Sources and Risks of Residual DNA
Mainstream Residual DNA Detection Technologies
Choosing High-Quality Residual DNA Testing Services
Our Professional Solutions – Creative Proteomics
In the rapidly evolving sectors of biopharmaceuticals, cell therapy, gene therapy, and forensic science, residual DNA testing is a critical quality and safety checkpoint. Residual DNA refers to trace amounts of host cell or vector DNA that may remain in final biological products after production and purification processes. Although often present in minuscule quantities, this DNA can raise safety concerns and regulatory red flags if not properly controlled and quantified.
Global regulatory frameworks—including ICH Q6B, FDA Guidance for Industry, and EMA quality guidelines—mandate stringent limits on residual DNA content to ensure that biological products meet safety, efficacy, and purity standards. As novel therapeutic modalities such as CAR-T therapies, mRNA vaccines, and viral vector-based treatments emerge, manufacturers must proactively address residual DNA risks to satisfy both compliance requirements and market expectations.
This article explores the biological origin, detection technologies, regulatory context, and strategic importance of residual DNA testing, ultimately guiding stakeholders toward informed decision-making in method selection and partner engagement.
Sources and Risks of Residual DNA
Primary Sources of Residual DNA in Biologics Manufacturing
Residual DNA in biologic products originates from the biological systems used during the upstream production process. These contaminants are often derived from:
- Host Cell Lines: Common production hosts include CHO (Chinese Hamster Ovary) cells, HEK293 (human embryonic kidney cells), E. coli, yeast (e.g., Pichia pastoris), and insect cells. Each cell line has a unique genomic profile, and their DNA can persist in the product despite purification efforts.
- Expression Vectors: Plasmids, viral vectors, or synthetic DNA constructs are introduced into host cells to drive recombinant protein expression. These vectors, or fragments thereof, can integrate into the host genome or remain episomal, leading to potential DNA contamination.
- Production By-products: Cell lysis, mechanical shear stress, and chemical agents used in bioprocessing may release nucleic acids into the supernatant. Even in well-controlled processes, the complex nature of bioreactors and large-scale fermentation increases the risk of DNA carryover.
- Downstream Processing Inefficiencies: While purification steps such as chromatography and filtration aim to remove process-related impurities, residual DNA can be retained due to suboptimal parameters, filter fouling, or insufficient clearance steps.
Biological and Regulatory Risks Associated with Residual DNA
Even trace amounts of residual DNA—typically measured in nanograms per dose—can carry significant biological and regulatory implications:
Oncogenicity and Horizontal Gene Transfer
Residual DNA may contain oncogenes, viral sequences, or antibiotic resistance genes from the host genome or expression plasmids. If integrated into a patient's cells, these sequences could theoretically lead to transformation events or disrupt native gene expression. Although the risk is low, regulators such as the FDA and EMA require a thorough risk assessment and justification for any residual DNA content.
Immunogenic Responses
Exogenous DNA, especially when of bacterial or viral origin, can be immunostimulatory. It may activate innate immune pathways such as toll-like receptor 9 (TLR9), potentially leading to inflammation or altered pharmacodynamics. This is especially critical in gene therapy, where immune responses can interfere with vector delivery and therapeutic efficacy.
Product Consistency and Reproducibility
Fluctuating levels of residual DNA across batches may signal process instability or inadequate purification. This variability undermines regulatory confidence and can trigger batch rejection or extensive revalidation, delaying development timelines.
Regulatory Non-Compliance and Market Delays
Authorities such as the FDA, EMA, and WHO enforce strict thresholds for residual DNA content—typically ≤10 ng/dose, with fragment size ≤200 base pairs for certain products. Exceeding these thresholds or failing to demonstrate validated control methods can result in:
- Clinical hold letters
- Post-approval manufacturing constraints
- Full or partial product recalls
- Market access delays or denials
Mainstream Residual DNA Detection Technologies
Accurate, sensitive, and reproducible detection of residual DNA is fundamental to regulatory compliance and product safety in biologics manufacturing. A variety of analytical platforms have been developed and refined to meet this challenge, each with distinct strengths and limitations. The choice of method often depends on product type, development stage, regulatory expectations, and throughput needs.
qPCR (Quantitative Polymerase Chain Reaction)
qPCR remains the gold standard for residual DNA quantification across the biopharmaceutical industry. It utilizes sequence-specific primers and fluorescent probes to amplify and quantify target DNA sequences in real time.
Advantages:
- Extremely high sensitivity (typically as low as 10 pg/mL)
- High specificity through primer design for host-specific genomes (e.g., CHO, E. coli)
- Scalable for in-process testing, lot release, and stability studies
- Widely accepted by regulatory agencies, with well-established validation protocols
Limitations:
- Requires careful assay design and optimization
- Subject to interference from complex biological matrices or residual purification agents
- Dependent on high-quality reference standards and controls
Digital PCR (dPCR)
Digital PCR partitions a sample into thousands to millions of nanodroplets or wells, each undergoing PCR amplification individually. It allows for absolute quantification without the need for standard curves.
Advantages:
- Ultra-sensitive detection of low-copy DNA molecules
- Absolute quantification increases reproducibility and accuracy
- High tolerance to inhibitors and matrix complexity
Use Cases:
- Low-dose gene therapy products
- Residual plasmid or viral DNA detection in early-stage vector development
- Confirmatory testing or cross-platform validation of qPCR results
Challenges:
- Higher operational costs
- Lower throughput compared to qPCR
- Specialized instrumentation and software required
Next-Generation Sequencing (NGS)
NGS allows for deep, unbiased profiling of DNA sequences in a sample. Although not routinely used for GMP release testing, it is increasingly utilized in exploratory studies and advanced characterization.
Strengths:
- Comprehensive identification of DNA species, including unknown or fragmented sequences
- Capable of detecting integration events or contamination sources
- High multiplexing capabilities
Limitations:
- Requires extensive bioinformatics infrastructure
- Longer turnaround times
- Higher cost per sample
Application:
- Investigational studies, product characterization, or root cause analysis
- Mapping foreign DNA integration or sequence mutation analysis in gene therapy platforms
Digestion-Free Residual DNA Assays
An emerging innovation in the field is digestion-free residual DNA testing, which eliminates the need for enzymatic digestion (e.g., with Benzonase) before amplification. This method enhances process efficiency and minimizes variability.
Benefits:
- Faster sample preparation and reduced hands-on time
- Less reagent dependency and fewer contamination risks
- Ideal for automation and high-throughput screening
Adoption Status:
- Gaining traction in QC labs for routine testing
- Suitable for biologics with low interfering excipients
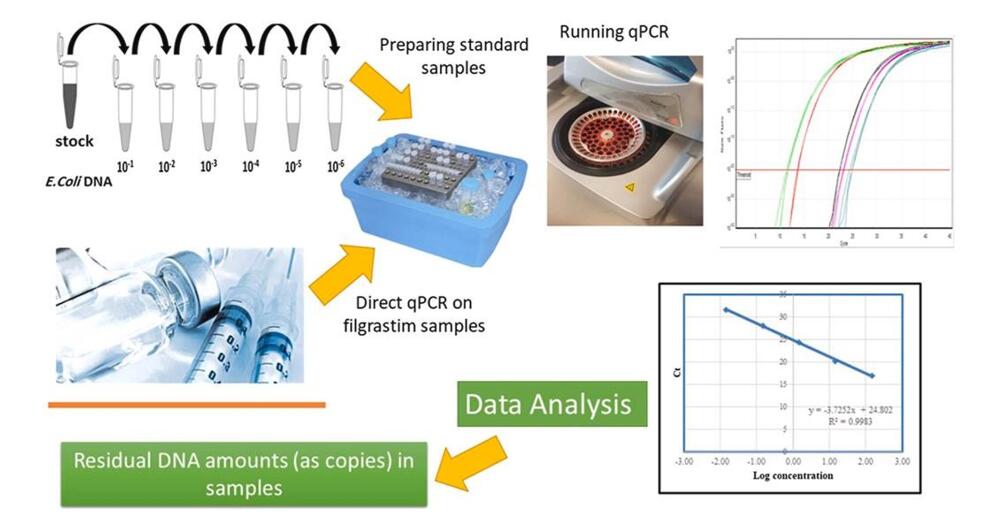
Direct quantitative detection of host cell residual DNA in recombinant Filgrastim by qPCR. (Gholizadeh-Hashjin, A., et al.; 2021)
Comparison of Technologies
| Method | Sensitivity | Specificity | Throughput | Turnaround Time | Best Fit For |
| qPCR | High | High | Moderate | 1–2 days | Routine QC, lot release, method validation |
| Digital PCR | Very High | Very High | Low | 2–3 days | Rare targets, confirmatory testing |
| NGS | Ultra High | High | High | 4–7 days | Research, profiling, root cause investigation |
| Digestion-Free qPCR | High | Moderate | Very High | <1 day | Automated workflows, batch testing |
Choosing High-Quality Residual DNA Testing Services
For organizations developing or commercializing biologics, the selection of a reliable residual DNA testing partner is not merely a matter of outsourcing—it's a strategic decision that affects compliance, product integrity, and regulatory milestones. Here's what to look for:
Analytical Performance and Method Suitability
Not all assays are created equal. A high-quality testing service provider should demonstrate:
- Sensitivity appropriate to product class: For instance, gene and cell therapies often require detection limits in the femtogram range, whereas monoclonal antibodies may need nanogram-level precision.
- Specificity toward relevant host systems: Primers and probes should be custom-designed and validated for each host cell type (e.g., CHO, HEK293, E. coli).
- Validated and compliant method protocols: Assays must be validated according to ICH Q2(R2), including parameters like linearity, LOD/LOQ, precision, and robustness.
Regulatory Accreditation and Quality Infrastructure
The credibility of a laboratory's results hinges on its compliance with recognized quality systems and certifications. Critical credentials include:
- GMP or GLP Certification: Demonstrates adherence to Good Laboratory Practices or Good Manufacturing Practices.
- ISO/IEC 17025 Accreditation: Ensures technical competence and quality assurance in testing and calibration.
- Audit-readiness: The lab should be prepared for inspections from FDA, EMA, or client QA teams, with complete documentation and traceability.
Technical Expertise and Method Customization
Top-tier service providers should offer more than just standardized assays—they should deliver tailored analytical strategies. This includes:
- Custom assay development for novel expression platforms or emerging host cells
- Technical consulting during IND, BLA, or MAA preparation
- Support for bridging studies during scale-up or process changes
The ability to adapt assays to the client's unique matrix, regulatory geography, and development phase can significantly shorten development timelines and enhance regulatory outcomes.
Operational Capabilities and Client Support
A strong testing partner must balance scientific rigor with operational excellence:
- Rapid turnaround times for time-sensitive studies (e.g., clinical trial lots or release testing)
- Flexible capacity to accommodate both high-throughput campaigns and low-volume R&D samples
- Transparent communication and project management from study initiation through reporting
Our Professional Solutions – Creative Proteomics
At Creative Proteomics, we offer specialized residual DNA testing services designed to meet the evolving analytical needs of biologics developers across the pharmaceutical, biotechnology, and advanced therapy sectors. Leveraging cutting-edge technologies and a team of experienced molecular biology experts, we provide comprehensive, high-sensitivity testing to support product development, process optimization, and regulatory readiness.
Advanced Testing Technologies for Accurate, Scalable Results
Our platform integrates multiple state-of-the-art methodologies to deliver flexible, high-throughput, and reproducible residual DNA quantification:
- qPCR-Based Assays: We employ host cell line-specific qPCR assays with high sensitivity and specificity, customized to the biological system used—whether CHO, HEK293, E. coli, or others. Our assays support detection levels down to picogram quantities, and are suitable for routine in-process monitoring and final product assessment.
- Digital PCR (dPCR): For applications requiring ultra-sensitive, absolute quantification—such as early-stage gene therapy development or low-dose biologics—our digital PCR platform enables detection of even single-copy DNA molecules in complex matrices.
- NGS-Enhanced Residual DNA Profiling: We offer exploratory services using next-generation sequencing for clients seeking deeper insights into DNA fragment origin, structure, or integration patterns. This can be particularly useful for investigational studies, contamination source tracking, or developing robust control strategies.
- Digestion-Free Residual DNA Detection: Our innovative digestion-free protocols streamline sample preparation, reduce turnaround time, and are well-suited to automated workflows—particularly beneficial for high-throughput environments or screening studies.
Whether supporting early discovery or late-stage development, our testing services are structured to adapt to changing project needs while maintaining scientific integrity and operational efficiency.
Client-Centric Delivery Model
We prioritize responsiveness, clear communication, and scientific partnership. Our turnaround times are competitive, and our client support team ensures proactive updates and technical engagement throughout the testing lifecycle.
References
Gholizadeh-Hashjin, Aiesheh, et al. "Direct quantitative detection of host cell residual DNA in recombinant Filgrastim by qPCR." Analytical Biochemistry 629 (2021): 114296. https://doi.org/10.1016/j.ab.2021.114296


