Verification of Synthetic Peptide Sequence
- Home
- Services
- Protein Sequencing
- Edman Based Protein Sequencing
- Verification of Synthetic Peptide Sequence
Service Details
The synthesis and application of peptides has become increasingly popular due to developments inin the fields of biotechnology and bioengineering and for a number of research purposes such as cancer diagnosis and treatment, antibiotic drug development, epitope mapping, production of antibodies, and vaccine design. With greater efficacy, safety, and tolerability in humans as compared to small molecules, peptides are drawing more attention as potential drug candidates targeting a wide variety of diseases. Edman degradation is commonly used for peptide sequencing both in solution and on solid support. The method is applicable to natural and non-natural α-amino acid residues located at the N-terminus, as long as their α-amino groups are unprotected. The usefulness of this method is based on the high yield of amino acid-PTH formation, suitable for identification using chromatography, electrophoresis or mass spectrometry.
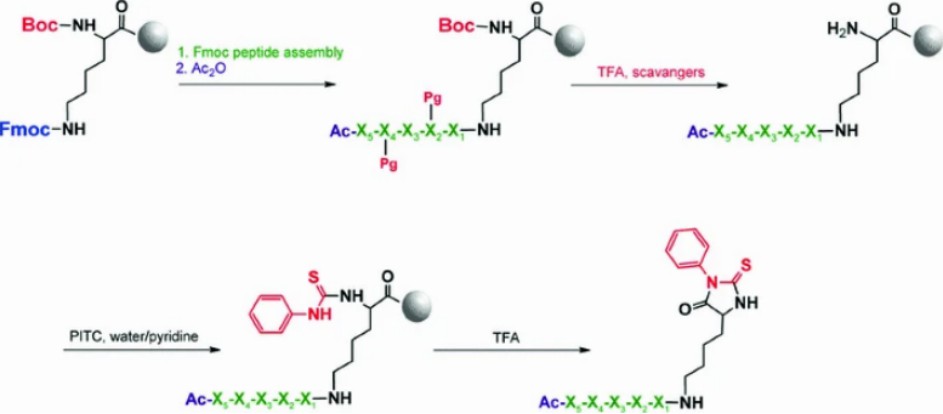 Fig. 1.
Proposed peptide cleavage method based on Edman degradation. (Bąchor R, et al., 2013)
Fig. 1.
Proposed peptide cleavage method based on Edman degradation. (Bąchor R, et al., 2013)
Synthetic peptide sequence verification refers to the determination of the amino acids that constitute the structural sequence of the peptide chain. Creative Proteomics provides comprehensive synthetic peptide sequence verification services to accelerate the research of your project.
At Creative Proteomics, for short peptides, our scientists generally employ Edman degradation, the standard for peptide sequencing, to analysis your amino acids sequence of synthetic peptide. Here, we have developed a complete sequencing process for you.
Ⅰ. Under alkaline conditions, PITC binds to the free amino group at the N-terminus of the protein.
Ⅱ. In acidic solution, the N-terminal residue is cleaved.
Ⅲ. The PITC-bound residues were converted into more stable PTH residues.
Ⅳ. The amino acid species was determined by on-line HPLC analysis according to the elution time.
If Edman degradation is not suitable for your synthetic peptide. Don't worry, here's another sequencing method to meet your demand. For synthetic peptides containing more than 70 amino acid residues, our scientists will use specific protein enzymes to digest them into small fragments according to the molecular weight of your synthetic peptide and the characteristics of amino acid composition, and then analyze the sequence of each small fragment. As the complement of the Edman degradation, the method ensure the smooth progress of the verification of synthetic peptide sequence services.
At Creative Proteomics, in addition to highquality results, you will also receive:
Creative Proteomics provides global customers with professional verification of synthetic peptide sequence service to speed up your peptide project journey. At Creative Proteomics, our highly qualified team works with our customers stand together on the front lines to help you solve tough research challenges. If you are interested in our services, please contact us immediately.
References
For research use only, not intended for any clinical use.