Biopharmaceutical Peptide Mapping Analysis Service
- Home
- Applications
- Biopharmaceutical Characterization Services
- Biopharmaceutical Identification Analysis Services
- Biopharmaceutical Peptide Mapping Analysis Service
Service Details
Peptide mapping, one of the important means for the quality control of biological drugs, has become one of the routine indicators for the quality standard control of protein and peptide drugs. It is used to compare the identity between recombinant and natural protein results, whether there are post-translational modifications and unknown amino acid variations in recombinant products, but also compare the peptide maps of different batches of products to verify the stability of the process. The principle of peptide mapping analysis is to use a variety of proteases to digest and identify target proteins according to the molecular weight and amino acid composition characteristics of proteins and peptides, then the entire amino acid of the protein can be obtained through splicing.
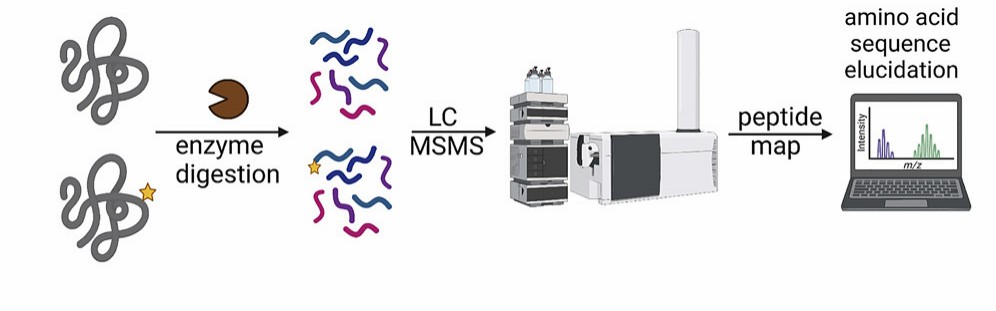 Fig. 1.
Peptide mapping analysis of AAV capsids using LC-MS/MS. (Lam AK, et al., 2022)
Fig. 1.
Peptide mapping analysis of AAV capsids using LC-MS/MS. (Lam AK, et al., 2022)
Peptide mapping is a key tool in pharmaceutical research and drug development to biomanufacturing and clinical testing, which has been used in many recombinant drugs as an important part of primary structure confirmation and quality control. As the world's leading provider of protein sequencing services, Creative Proteomics has developed a peptide mapping analysis platform based on liquid chromatography and tandem mass spectrometry (LC-MS/MS) for biological drug primary structure confirmation.
Our peptide mapping service can be used to analyze the sequence of biopharmaceuticals and confirm the complete expression of recombinant proteins in compliance with ICH Q6B.
It is worth noting that some residues such as isoleucine and leucine have the same mass, so mass spectrometry programs cannot distinguish between them. For studies requiring complete primary amino acid sequencing, our Edman degradation is best choice to analyze the pooled peptides.
In addition, some biopharmaceuticals should undergo de novo amino acid sequencing, of which sequences cannot be found in protein databases.
 Fig. 2. Workflows of our
biopharmaceutical peptide mapping analysis service.
Fig. 2. Workflows of our
biopharmaceutical peptide mapping analysis service.
Peptide mapping enable you to meet the structural characterization requirements of the ICH Q6B guidelines, including determining the primary structure of proteins, assessing post-translational modifications (PTMs), and analyzing product-related impurities. Our biopharmaceutical peptide mapping analysis can provide you with a large amount of information on the following protein primary structures, including but not limited to.
Creative Proteomics is a reliable biopharmaceutical partner. Our professional team can provide customers with comprehensive biopharmaceutical peptide mapping information, and realizing the analysis of protein advanced structure and modification sites. We will provide you with detailed experimental procedures and data reports. Please contact us immediately If you are interested in our services.
References
For research use only, not intended for any clinical use.