N-Glycosylation Site Analysis Service
- Home
- Applications
- Proteomics Analysis Services
- Glycoproteomics Analysis Services
- N-Glycosylation Site Analysis Service
Service Details
Protein N-glycosylation plays an important role in physiological and pathological processes. Characterizing site-specific N-glycosylation, including N-glycan macroscopic heterogeneity (glycosylation site occupancy) and microscopic heterogeneity (site-specific glycan structure), is important for understanding glycoprotein biosynthesis and function.
N-glycosylation is a post-translational modification of proteins involved in a variety of physiological and pathological processes. N-glycosylation site occupancy reflects the ratio of N-glycosylation in a protein, a phenomenon that in turn reveals the availability of sites, substrate concentrations, and enzyme kinetics in the ER. The properties of glycoproteins are largely affected by site occupancy, and defects in site occupancy may be detrimental to physiological functions. Therefore, accurate determination of site occupancy is important significance to fully understand the impact of protein glycosylation on human health.
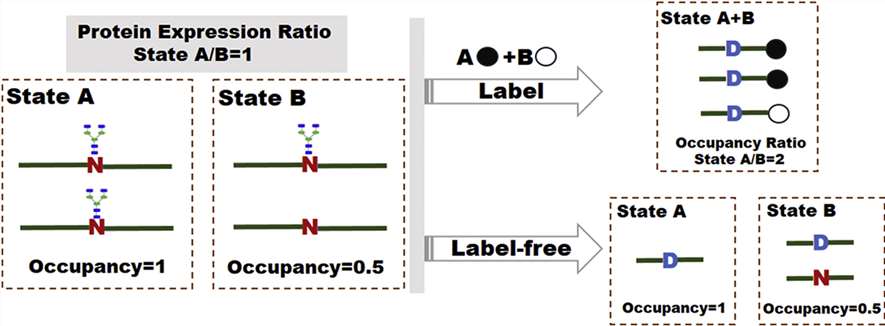 Figure
1. MS-based labeling and label-free technologies for quantification of N-glycosylation site occupancy. (Zhang S,
et al., 2017)
Figure
1. MS-based labeling and label-free technologies for quantification of N-glycosylation site occupancy. (Zhang S,
et al., 2017)
Liquid chromatography-mass spectrometry (LC-MS)-based quantification has been widely used and provides fairly advanced quantification of N-glycosylation site occupancy in a sample. At Creative Proteomics, we provide tools for the quantification of N-glycosylation sites for Labeled and label-free techniques for glycosylation site occupancy. SILAC, TMT, dimethylation, and iTRAQ are commonly used to introduce "heavy" and "light" isotope or isobaric labels into samples, while label-free methods are based on spectral counting or peptide ionic strength.
Creative Proteomics provides the following N-glycosylation site analysis services, including but not limited to.
A significant advantage of the labeling approach is the ability to efficiently analyze differentially labeled samples simultaneously in a single run. We can offer trypsin-catalyzed 18 O, SILAC and iTRAQ labeling methods for N-glycosylation site occupancy analysis. Isotope-encoded glycosylation site-specific tags (IGOTs) are available for large-scale N-glycosylation site analysis.
A general strategy for the analysis of variable N-glycosylation sites in glycoproteins includes the following procedures.
Label-free quantification of N-glycosylation site occupancy is usually based on peptide ion intensity or peak area. Its significant advantage is the simple workflow.
A general strategy for the analysis of variable N-glycosylation site occupancy in glycoproteins includes the following procedures.
The ICH Q6B guideline requires comprehensive characterization of glycoprotein glycosylation. With years of experience and an experienced scientific team, Creative Proteomics provides diverse and systematic N-glycosylation site analysis services. Additionally, we can provide fully custom project designs to meet any specific requirements. Our efficient service and accurate results save clients time and money. If you are interested, please contact us or send us an inquiry directly.
References
For research use only, not intended for any clinical use.