Biopharmaceutical Glycosylation Site Analysis Service
- Home
- Applications
- Biopharmaceutical Characterization Services
- Biopharmaceutical Variation Analysis Services
- Biopharmaceutical Glycosylation Site Analysis Service
Service Details
Glycosylation affects the spatial conformation, biological activity, transport, and localization of proteins, but also plays a vital role in specific biological processes such as molecular recognition, cell communication, and signal transduction. As one of the important structural modifications that occur during the production of many protein-based drugs, glycosylation plays an important role in ensuring the correct structure and function of most biotherapeutic proteins, even small changes in glycan composition, structure, or position can have dramatic effects on drug safety and efficacy. In the context of ongoing biopharmaceutical innovations, accurate and detailed analysis of protein glycosylation is of critical importance to biopharmaceutical companies and government regulators alike. At present, Mass spectrometry (MS) is a promising technique for high-quality analytical characterization of glycosylation, from early drug development to final batch release.
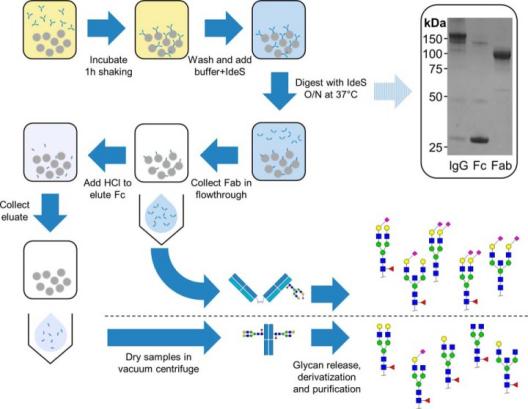 Fig.
1. Workflow of IgG Fab and Fc glycosylation analysis. (Bondt A, et al., 2014)
Fig.
1. Workflow of IgG Fab and Fc glycosylation analysis. (Bondt A, et al., 2014)
Glycosylation has profound effects in ensuring the correct structure and function of most biotherapeutic proteins. As a forward-looking company and a market leader in the field of mass spectrometry, Creative Proteomics has successfully completed many challenging projects in the analysis of glycosylation sites in biopharmaceuticals. Here, we provide one-stop glycosylation analysis services for your biopharmaceutical research and development.
Our biopharmaceutical glycosylation analysis solution can perform precise positioning analysis of N-glycosylation and O-glycosylation of therapeutic glycoproteins, and can also provide glycan chain composition information. Data analysis adopts Byonic, the current international mainstream glycosylation data analysis software, which greatly improves the reliability of the identification results of glycosylation modification sites.
To obtain all the glycosylation information required for the complete structural characterization of therapeutic glycoproteins, Creative Proteomics uses the following protocol for analysis.
To provide further structural details, Creative Proteomics also provides services such as glycan analysis, glycosylation site occupancy analysis, and monosaccharide analysis. This is very important because the production of biosimilars must match the innovator drug in structure.
The ICH Q6B guideline requires comprehensive characterization of biopharmaceutical samples glycosylatio. Our glycosylation site analysis service can help you with the following, but not limited to.
Creative Proteomics is a reliable biopharmaceutical partner. Our professional team can provide a wide range of glycosylation site analysis services to our clients worldwile. Our services guarantee accurate and reliable results, at quick turnaround time! If you would like more information about specific aspects of our services, feel free to contact us.
References
For research use only, not intended for any clinical use.