Biopharmaceutical N/C-Terminal Sequencing Service
- Home
- Applications
- Biopharmaceutical Characterization Services
- Biopharmaceutical Identification Analysis Services
- Biopharmaceutical N/C-Terminal Sequencing Service
Service Details
The N/C terminal sequence is an important structural and functional part of proteins and polypeptides, and even plays a decisive role in the biological functions of proteins. The commonly methods for N-terminal sequencing are Edman degradation and mass spectrometry, and the main methods for protein C-terminal sequencing are carboxypeptidase method, chemical method and tandem mass spectrometry. However, there are its own advantages and disadvantages about each of these methods. For example, the sequencing problem of N-terminal blocking (circularization, blocking) is not solved, and an exact signal of the modified protein cannot given by Edman degradation method. Additionaly, the purity of the protein or polypeptide is required to be above 95%. On the contrary, Mass Spectrometry locate the post-translational processing or chemical modification of side chain groups, and make up for the defect that the traditional Edman degradation method is not suitable for N-terminal blocking, which is complementary to ensure the smooth progress of N and C terminal sequencing.
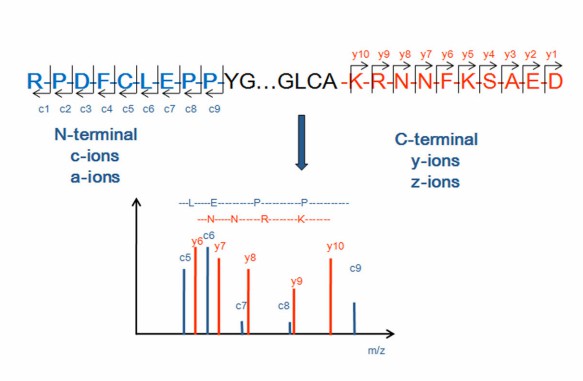 Fig. 1. Principles of ISDMALDI MS.
(Ejvind Mort, et al., 2001)
Fig. 1. Principles of ISDMALDI MS.
(Ejvind Mort, et al., 2001)
It is a regulatory requirement to confirm the sequence of protein and examine the termini for any variation that may exist. Creative Proteomics, as the world's leading provider of protein sequencing services, provides you with high-quality one-stop biopharmaceutical N/C-terminal sequencing services with first-class experimental platforms and mature technical means.
To decode the N/C-terminal sequence of biopharmaceuticals, Creative Proteomics' scientists use the following N/C-terminal sequencing protocol:
 Fig. 2. Workflow of our
biopharmaceutical N/C-terminal sequencing service.
Fig. 2. Workflow of our
biopharmaceutical N/C-terminal sequencing service.
Our mass spectrometry-base N-terminal sequencing service is free from interference from N-terminal blocking and modification sites. Amino acid sequence information is generated from chromatographically separated peptides, providing much useful structural information as part of such characterization studies.
There is no fully analogous method to definitive C-terminal sequencing of biopharmaceuticals like Edman chemistry. To assess the C-terminus of biopharmaceutical, Creative Proteomics' scientists offer analysis by carboxypeptidase hydrolysis in addition to mass spectrometry sequencing.
First, Our team of experts carry out the kinetic experiment of carboxypeptidase to determine the appropriate enzyme concentration and reaction time, so that the released amino acids are mainly C-terminal amino acids. According to the relationship between the amount of released amino acids (moles) and the reaction time, the peptide chain can be identified C-terminal amino acid sequence. The specific workflow is as follows.
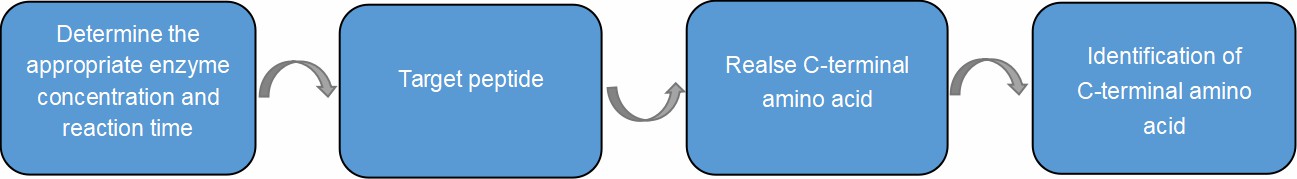 Fig. 3. Workflow of
Carboxypeptidase hydrolysis method.
Fig. 3. Workflow of
Carboxypeptidase hydrolysis method.
Creative Proteomics is a reliable biopharmaceutical partner. Our professional team can provide customers with comprehensive biopharmaceutical protein N/C-terminal sequence sequencing information, and realize the analysis of protein advanced structure and modification sites. We will provide you with detailed experimental procedures and data reports such as the final N/C-terminal sequence information analysis report. Please contact us immediately If you are interested in our services.
References
For research use only, not intended for any clinical use.