Antibody Deamidation and Isomerization Analysis Service
- Home
- Applications
- Biopharmaceutical Characterization Services
- Biopharmaceutical Variation Analysis Services
- Antibody Deamidation and Isomerization Analysis Service
Service Details
Asparagine (Asn) deamidation and aspartic (Asp) isomerization are two of the most common degradation reactions that occur during long-term storage of mAb products, and they can occur at different stages of the mAb production process. Product heterogeneity resulting from uncontrolled degradation due to deamidation and isomerization can complicate manufacturing consistency, hampering long-term mAb functionality. Therefore, drug candidates prone to degradation should be identified early in the drug development process to adjust manufacturing and formulation processes accordingly or redesign problematic drug candidates to eliminate such hotspots.
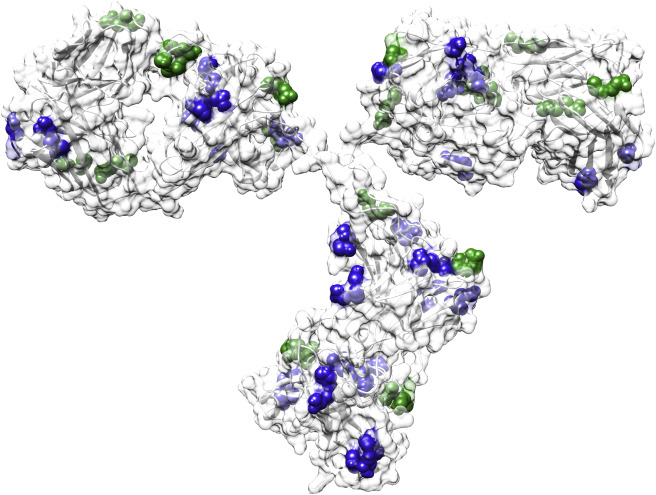 Fig.
1. Potential chemical degradation sites (deamidation and isomerization) in an antibody (PDB: 1IGT). (Kuroda D,
et al., 2020)
Fig.
1. Potential chemical degradation sites (deamidation and isomerization) in an antibody (PDB: 1IGT). (Kuroda D,
et al., 2020)
Accurate assessment of chemical modifications such as asparagine deamidation and aspartate isomerization is an important part of the comprehensive characterization of therapeutic monoclonal antibodies (mAbs). When located in complementarity-determining regions (CDRs), these modifications lead to loss-of-function, affecting product efficacy and safety, and leading to the designation of the modification as a critical quality attribute.
As a quality partner of the biopharmaceutical industry. Creative Proteomics has successfully completed many challenging projects in antibody analysis. You have reason to believe that we are the ideal partner for your project.
Creative Proteomics offers a wide range of services for the characterization of therapeutic proteins. Whether you're looking for a custom or off-the-shelf solution, Creative Proteomics has over years of expertise in delivering unique and powerful assays to help accelerate your treatment plan.
Here, our experienced scientists have developed an advanced platform for antibody deamidation and isomerization analysis. Our antibody deamidation and isomerization analysis services are based on peptide mapping analysis. To accurately characterize antibody deamidation and isomerization, Creative Proteomics' expert team uses the following protocol.
It is noted that to enhance the characterization of sequence responsibility, liquid chromatography coupled with online tandem mass spectrometry (LC-MS/MS) is required to provide site mapping of chemical modifications, as well as direct, confirmatory sequencing for peptide identification. Proteolytic digestion is usually performed overnight at the optimal pH for the enzyme to ensure complete digestion of the protein.
Creative Proteomics is a reliable biopharmaceutical partner. Our professional team can provide professional, systematic antibody deamidation and isomerization analysis service for our clients worldwile. Our services guarantee accurate and reliable results, at quick turnaround time! If you would like more information about specific aspects of our services, feel free to contact us.
References
For research use only, not intended for any clinical use.