Disulfide Bond Analysis Service
- Home
- Applications
- Proteomics Analysis Services
- Protein Post-Translational Modification Analysis Services
- Disulfide Bond Analysis Service
Service Details
A disulfide bond is a covalent bond formed between the sulfur atoms of two cysteines and is a common post-translational modification in proteins. Their presence ensures that the protein folds correctly, remains stable, maintains normal function, etc. As a critical quality attribute of proteins, the analysis of disulfides is critical for the development and identification of biotherapeutics.
Analytical methods to characterize the integrity of protein disulfide bond patterns have continued to evolve and today range from classical electrophoretic methods such as SDS-PAGE and CE, absorbance spectroscopy for thiol-reactive labeling and Edman sequencing, to state-of-the-art MS analysis. Notably, mass spectrometry is the most prominent tool for protein disulfide bond analysis today. Most LC-MS/MS methods for protein sample analysis are based on bottom-up approaches. Briefly, the technique requires proteolytic digestion of relevant proteins, followed by identification based on the quality of the resulting peptides, often relying on database comparisons. Bottom-up MS is the most widely used method for disulfide bond analysis compared to the limitations of other methods, such as high-purity sample requirements and low throughput.
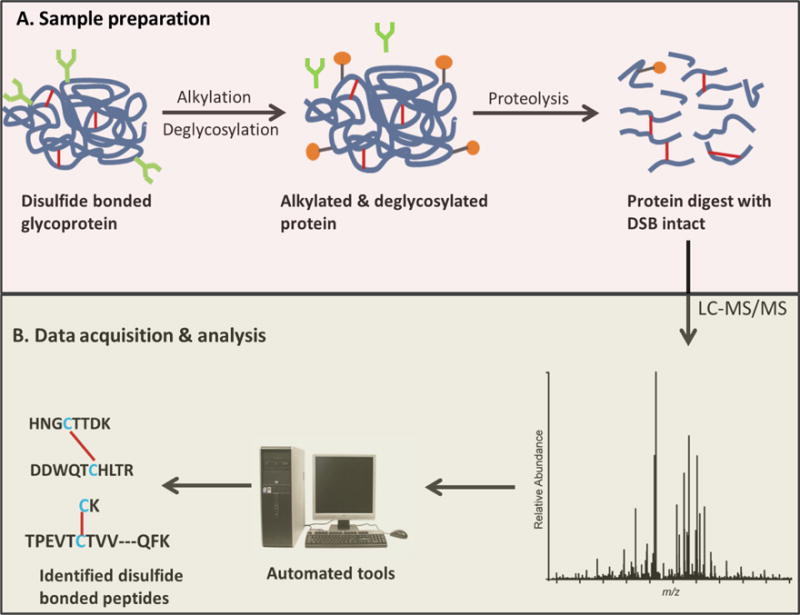 Fig. 1. Disulfide bond analysis workflow.
(Lakbub J C, et al., 2018)
Fig. 1. Disulfide bond analysis workflow.
(Lakbub J C, et al., 2018)
Creative Proteomics has extensive experience in protein characterization by mass spectrometry, especially protein sequence analysis. At Creative Proteomics, our scientists have introduced a comprehensive protein disulfide analysis solution to help you with your research, including normal, closely spaced, and mismatched disulfide bonds analysis, etc.
According to ICH Q6B guidelines, the biotherapeutics should determine the number and location of any free sulfhydryl and/or disulfide bonds whenever possible. Peptide mapping (under reducing and non-reducing conditions), mass spectrometry, or other appropriate techniques may be useful for this assessment. We introduce a highly sensitive HPLC-MS/MS platform for this purpose to analysis disulfide bonds in multiple samples from different sources.
Here, we provide one-stop disulfide bond analysis services, including sample preparation, protein purification, enzymatic hydrolysis, LC-MS/MS identification and bioinformatics analysis.
Simple disulfide bond analysis workflow:
Our laboratory technicians have extensive technical experience in elucidating disulfide bond structures. Our featured services include but not limited to:
Here, we provide a comprehensive service for disulfide bond analysis according to ICH Q6B guidelines, our service features are:
Thanks to our powerful mass spectrometry sequencing platform, Creative Proteomics provides a one-stop shop for complex disulfide bonds. Our experienced scientists work with you to develop tailor-made analytical solutions. All you need to do is send us your samples, communicate your research goals with our experts, and we'll go through the next steps and provide you with a full technical report. Please feel free to contact us with any questions regarding disulfide bond analysis.
References
For research use only, not intended for any clinical use.