IgE Antibody Sequencing
- Home
- Services
- Antibody Sequencing
- Mass Spectrometry Based Antibody Sequencing
- Antibody Full Amino Acid Sequencing
- IgE Antibody Sequencing
Service Details
Immunoglobulin E (IgE), a class of cytophilic antibodies with a δ chain, is the main antibody involved in the regulation of the pathogenesis of allergic rhinitis, allergic asthma and eczema. Among the five types of immunoglobulins, IgE immunoglobulin is the least content in normal human serum with the most unstable to heat, the shortest half-life, the highest decomposition rate and lowest synthesis rate. It is mainly synthesized by B cells in the lamina propria lymphoid tissue of the respiratory tract and digestive tract, and is a mediator of allergic reactions. IgE molecule differs most significantly from that of IgG in respect to the "additional" heavy chain constant domain and the absence of a hinge region in the ε-chain. The six domains comprising the IgE-Fc, a dimer of Cε2-Cε3-Cε4 domains, are evolutionarily more ancient than the four-domain IgG-Fc. IgE-Fc resembles the Fc structure of IgM, the most primitive antibody class, and the Fc domains of avian IgY, the ancestor of IgE and IgG.
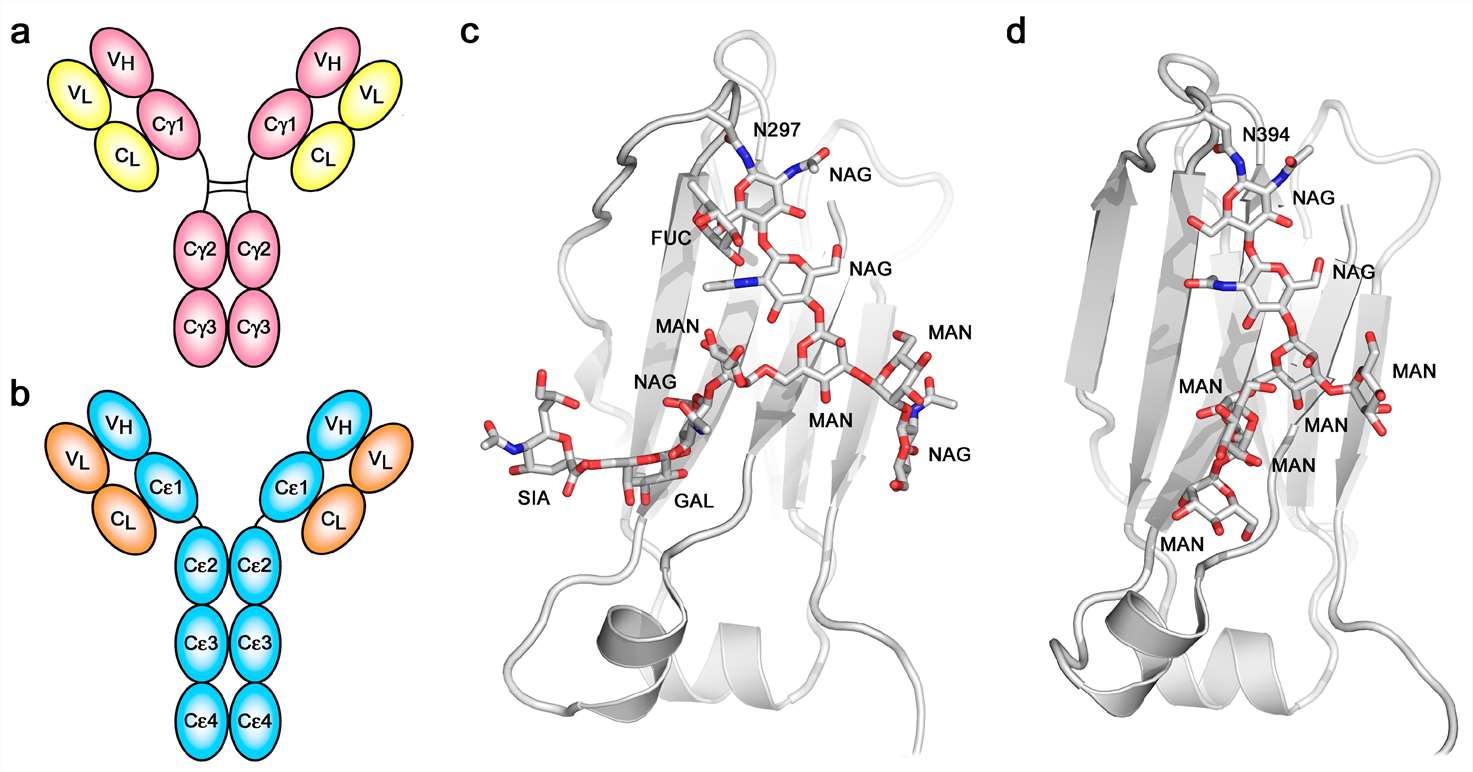 Fig. 1. Overall structure and glycosylation.
(Sutton B J, et al., 2019)
Fig. 1. Overall structure and glycosylation.
(Sutton B J, et al., 2019)
Creative Proteomics provides a complete IgE antibody sequencing service. Similar to other antibody sequencing, our IgE Antibody Sequencing service also is achived by using de novo sequencing methods on the basis of a powerful mass spectrometry platform. De novo sequencing is our proprietary protein sequencing technology that has been developed for many years, and we guarantee 100% sequence coverage of the detection results.
Complete IgE de novo sequencing is a very complex process, including multiple different protease hydrolysis, a series of chemical derivatization reactions, LC-MS data retrieval, bioinformatics analysis and manual mapping analysis with more than a dozen proprietary software, etc.
At Creative Proteomics, with decades of sequencing experience, our talented scientists have developed a complete and high quality sequencing process, it roughly includes the following steps:
Ⅰ. Proteolytic digestion and chemical derivatization reactions.
Ⅱ. LC-MS data acquisition.
Ⅲ. Database search and peptide de novo sequencing.
Ⅳ. Preliminary sequence splicing.
Ⅴ. Sequence supplementation.
Ⅵ. Sequence verification.
At Creative Proteomics, our de novo sequencing technology is also applicable to other proteins. However, some of these steps need to be changed depending on the protein properties.
The protein amount is preferably greater than 1mg (purity > 90%) due to the need for multiple different protease hydrolysis during the sequencing process.
Creative Proteomics provides professional IgE antibody sequencing services for global customers. Our team has successfully sequenced thousands of antibodies to help researchers decode their antibodies in a fraction of the time. Therefore, please contact us if you are interested in our antibody sequencing services.
References
For research use only, not intended for any clinical use.