Antibody C-Terminal Variation Analysis Service
- Home
- Applications
- Biopharmaceutical Characterization Services
- Biopharmaceutical Variation Analysis Services
- Antibody C-Terminal Variation Analysis Service
Service Details
Antibody N-terminal variation and C-terminal variation are the main forms of antibody drug terminal variation. For the C-terminal variation, sequence truncation is common, such as the deletion of C-terminal lysine (i.e., K deletion) in the heavy chain of antibody protein drug molecules leads to changes in the sequence of the C-terminal polypeptide molecule after enzymatic digestion. The two C-terminal polypeptide molecules quantitative results (with or without K deletion) can reflect protein molecules, which also illustrates the importance of C-terminal variation. In quality control, qualitative and quantitative analysis methods for such phenomena need to be established. Methods such as IEF, cIEF, ion-exchange chromatography, and LC-MS are commonly used to analyze the variation of C-terminal lysine. At present, digesting the sample with trypsin and then analyzing the sample by LC-MS is a relatively mature and common way to detect the proportion of antibody C-terminal K deletion.
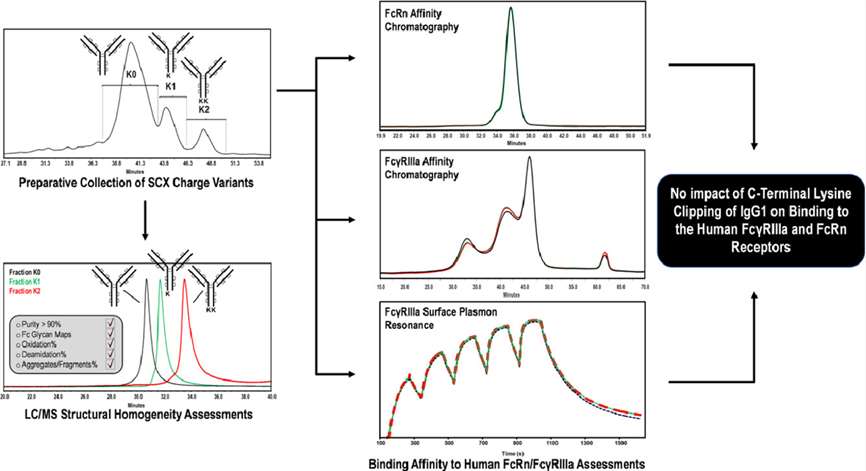 Fig. 1. C-terminal lysine
clipping of IgG1. (Faid V, et al., 2021)
Fig. 1. C-terminal lysine
clipping of IgG1. (Faid V, et al., 2021)
C-terminal K deletion determination is mandatory in ICH Q6B guidelines for testing procedures of biotechnology products. As a forward-looking company and a market leader in mass spectrometry, Creative Proteomics has successfully completed many challenging projects in the ratio analysis of antibody C-terminal K deletions. Here, our professional scientists used LC-MS for antibody C-terminal variation analysis, including detection of the proportion of antibody C-terminal K deletions and other types of truncations at antibody C-terminus.
In order to obtain the C-terminal variation analysis required for the complete structural characterization of therapeutic proteins, Creative Proteomics' main method for determining the C-terminal K deletion is based on SIM scanning in mass spectrometry identification.
Throughout the analysis cycle, Creative Proteomics' scientists first digest the antibody of interest with trypsin, and the peptides in the digestion mixture are separated by RP-HPLC and then detected in mass spectrometry. One point of concern is that specific and singly charged ions corresponding to the C-terminal peptide will be extracted in tandem mass spectrometry. The acquired MS and MS/MS spectra provided all the information of the lysine variants.
With our extensive mass spectrometry experience, you can also expect a comprehensive analysis report, including:
In addition to C-terminal variation analysis with extensive expertise in PTM analysis, Creative Proteomics can apply orthogonal analysis methods to identify a series of PTMs, including glycosylation, N-terminal cyclization, deamidation, etc.
Creative Proteomics is a reliable biopharmaceutical partner. Our professional team can provide antibody C-terminal variation analysis service for global customers. We will provide clear, comprehensive written reports, recommendations and agreements, as well as customized services to help clients solve analytical and technical problems. If you would like more information on a specific aspect of our services, please do not hesitate to contact us, we will be happy to answer any questions.
References
For research use only, not intended for any clinical use.