Biopharmaceutical N-Terminal Sequencing Service
- Home
- Applications
- Biopharmaceutical Characterization Services
- Biopharmaceutical Identification Analysis Services
- Biopharmaceutical N-Terminal Sequencing Service
Service Details
The N-terminal sequence composition has a great influence on the overall biological function of biopharmaceuticals (proteins, antibodies, and vaccines). N-terminal sequencing analysis of biopharmaceuticals helps to identify their higher-order structures while interpreting them to reveal their biological functions. For protein drugs, N-terminal sequencing analyze protein modification sites, such as the N-terminus of proteins and peptide drugs, analyze the position of artificial modifications such as cyclization modification and methylation modification to improve its degradation stability and prolong drug efficacy. In addition, multiple subunits, such as reduced monoclonal antibodies, can also be sequenced after separation by SDS-PAGE. Edman degradation N-terminal sequencing analysis is widely used as a simple biological product identification test, complements the confirmation of impurity sequence by mass spectrometry, and can simplify the identification of leucine-isoleucine, which is difficult for mass spectrometry.
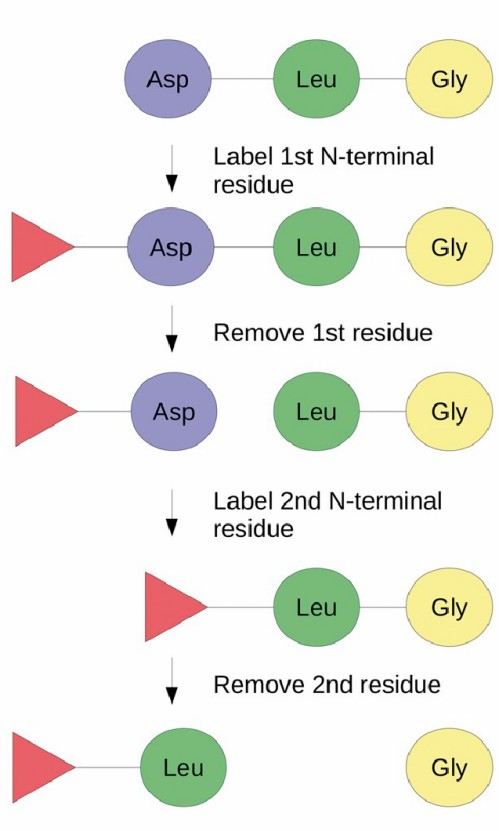 Fig. 1. Automated Edman peptide
sequencing. (Gauthier J, et al., 2019)
Fig. 1. Automated Edman peptide
sequencing. (Gauthier J, et al., 2019)
The analysis and confirmation of the N-terminal sequence of protein and polypeptide drug molecules is an important link in the quality control of the pharmaceutical industry. In particular, the ICH Q6B guideline requires the N-sequence information of protein (peptide) drugs. Creative Proteomics, as the world's leading provider of protein sequencing services, provides you with high-quality one-stop biopharmaceutical N-terminal sequencing services with first-class experimental platforms and mature technical means.
Our Edman degraded N-terminal sequencing technology can provide N-terminal sequencing analysis methods for biopharmaceutical proteins or other biological reagents, complementing mass spectrometry sequencing to obtain comprehensive N-terminal sequence analysis information.
To decode the N-terminal sequence of biopharmaceuticals, Creative Proteomics' scientists use the following N-terminal sequencing protocol:
N-terminal sequence analysis has many different applications in the development of biopharmaceuticals, including but not limited to.
Creative Proteomics is a reliable biopharmaceutical partner. Our professional team can provide customers with comprehensive biopharmaceutical protein N-terminal sequence information, and realizing the analysis of protein advanced structure and modification sites. We will provide you with detailed experimental procedures and data reports such as the final N-terminal sequence information analysis report. Please contact us immediately If you are interested in our services.
References
For research use only, not intended for any clinical use.