N-Glycan Analysis Service
- Home
- Applications
- Proteomics Analysis Services
- Glycoproteomics Analysis Services
- N-Glycan Analysis Service
Service Details
Protein glycosylation, the addition of monosaccharide or oligosaccharide chains (glycans) to peptide backbones, including proteins, lipids, or other organic molecules, is a common post-translational modification (PTM) that confers various biological function. Glycosylation analysis is challenging due to the complexity and isobaric nature of linked glycans.
N-glycosylation, in which the glycan is attached to the nitrogen side chain of asparagine, is the most widely known form of glycosylation in biotherapeutics. N-glycans have been reported to regulate a variety of biological processes, such as ligand-receptor interactions, immune responses, protein secretion and transport, and plays an important role in a variety of diseases. Therefore, analyzing the link between N-glycan structure and its function is an important research area in elucidating molecular mechanisms involved in pathogenesis, monitoring diagnosis, prognosis, and biopharmaceutical development and quality control.
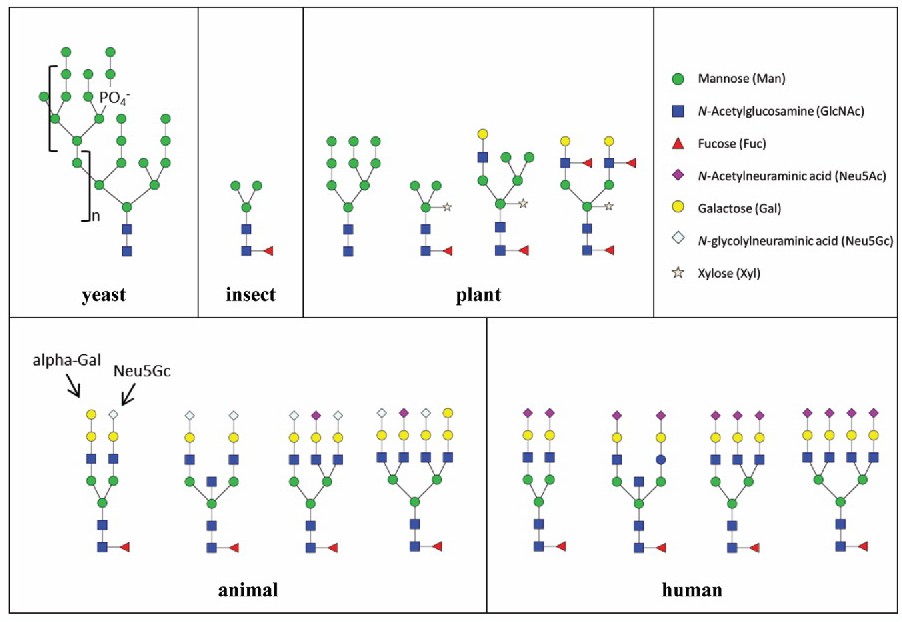 Fig. 1.
Examples of N-glycans found in the commonly used expression systems. (Zhang L, et al., 2016)
Fig. 1.
Examples of N-glycans found in the commonly used expression systems. (Zhang L, et al., 2016)
LC-MS-based N-glycan analysis is now the mainstream analysis method. Creative Proteomics provides the following N-glycan analysis services to analyze glycans and their species, including but not limited to.
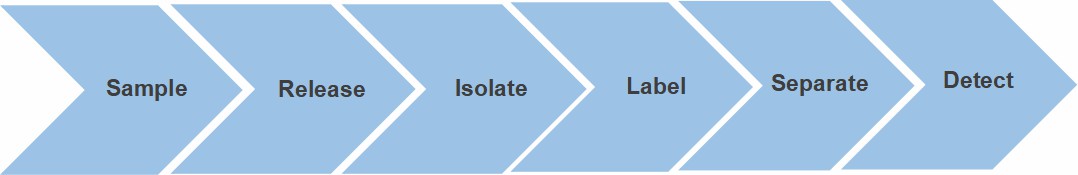 Fig. 2. Workflow for N-glycan profiling
service.
Fig. 2. Workflow for N-glycan profiling
service.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is a new type of ionization biological mass spectrometry developed in recent years. It is suitable for high-throughput screening of proteins or other biological macromolecules with high sensitivity for the structural characterization of glycosylated compounds. After the N-glycans of the sample are released using N-glycosidase A or F enzymatic reactions, the released glycans can be rapidly analyzed by MALDI-TOF MS, where neutral and sialylated glycans are analyzed simultaneously in positive ion mode. We offer the following two analysis methods.
Glycan permethylation with methyl iodide was used to improve ionization efficiency and stabilize sialylated glycans, then N-glycans were detected by MALDI-TOF MS.
Stabilizes sialic acids by linker-specific sialic acid esterification, focusing on the differentiation of α-2,3 and α-2,6 sialic acid linkages and NeuNAc and NeuNGc residues in the N-glycan profile.
Hydrophilic interaction chromatography (HILIC) can be used for N-glycan analysis. [4-amino-N-(2-diethylaminoethyl) benzamide]-labeled N-glycans (procainamide-labeled N-glycans) were analyzed by fluorescence and mass spectrometry detection. Compared with the traditional labeling methods of 2-aminobenzamide (2-AB) and 2-aminobenzoic acid (2-AA), we provide procainamide [4-amino-N-(2-diethylaminoethyl) benzamide]-labeled N-glycans show enhanced ESI ionization efficiency and fluorescence intensity, and thus improve identification of low abundance N-glycans.
*Note: Creative Proteomics can also perform glycan studies at the glycopeptide or glycoprotein level, providing you with complete N-glycan characterization services for glycosylation.
ICH Q6B guidelines require comprehensive characterization of glycosylation of glycoproteins. With years of experience and an experienced scientific team, Creative Proteomics provides high-quality N-glycan analysis services. Additionally, we can provide fully custom project designs to meet any specific requirements. If you are interested, please contact us or send us an inquiry directly.
References
For research use only, not intended for any clinical use.