O-Glycosylation Site Analysis Service
- Home
- Applications
- Proteomics Analysis Services
- Glycoproteomics Analysis Services
- O-Glycosylation Site Analysis Service
Service Details
Unlike N-glycans, O-glycans are smaller, highly diverse branched-chain carbohydrate molecules that attach to different protein structures. Site-specific O-glycosylation analysis is the key to exploring glycoprotein structure-function relationships. It determines whether predicted O-glycosylation sites are modified and the specificity of O-glycosylation at each of these sites. Glycosylation site analysis can not only intuitively obtain information on glycosylation changes of many disease markers, but also make further sugar chain structure analysis more direct and convenient. In recent years, mass spectrometry (MS)-based methods have proven to be the core technology for site-specific O-glycosylation analysis due to their high resolution and sensitivity. The different glycoforms typically released from proteins are identified as individual Glycans and their relative proportions, and site-specific characterization of glycosylation was subsequently performed.
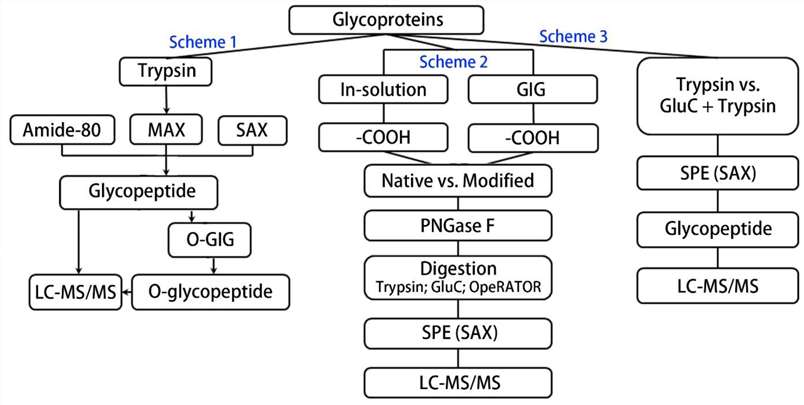 Fig. 1.
Schematic flowchart of sample processing for site-specific O-glycosylation analysis. (Yang S, et al., 2021)
Fig. 1.
Schematic flowchart of sample processing for site-specific O-glycosylation analysis. (Yang S, et al., 2021)
A number of features make the identification of O-linked sites difficult. 1) There is no known amino acid consensus sequence for O-linked glycans. 2) There is often significant heterogeneity in O-glycosylated protein regions, both in the number of glycans and in their degree of occupancy. Creative Proteomics utilizes state-of-the-art MS platforms and other proteomic technologies to provide accurate and reliable O-linked glycosylation site analysis services to identify genuine O-glycosylated peptides.
Creative Proteomics provides the following O-glycosylation site analysis service, including but not limited to.
O-glycans are released by enzymatic or chemical treatment, followed by subsequent characterization.
Glycoproteins are digested into peptides and then glycan-attached peptides are analyzed.
The ICH Q6B guideline requires comprehensive characterization of glycoprotein glycosylation. With years of experience and an experienced scientific team, Creative Proteomics provides diverse and systematic O-glycosylation site analysis services. Additionally, we can provide fully custom project designs to meet any specific requirements. Our efficient service and accurate results save clients time and money. If you are interested, please contact us or send us an inquiry directly.
References
For research use only, not intended for any clinical use.