O-Glycan Modification and Modification Site Analysis Service
- Home
- Applications
- Proteomics Analysis Services
- Glycoproteomics Analysis Services
- O-Glycan Modification and Modification Site Analysis Service
Service Details
O-glycosylation occurs through the initial monosaccharide linkage of mannose, galactose, fucose, glucose, xylose, or N-acetylglucosamine. These glycosylation events, commonly found in secreted and membrane-bound proteins, occur in the Golgi apparatus, and this process is often referred to as mucin-type O-glycosylation, which governs protein localization and trafficking, protein solubility, antigenicity and cell-cell interactions.
Unlike N-glycosylation, there exists a general enzyme that can deglycosylate various common core structures of proteins. In contrast, O-glycans release must be done chemically. Under alkaline conditions, the O-glycosidic bonds between reducing glycans and serine/threonine residues are unstable and easily hydrolyzed. The α-carbon adjacent to the sugar-binding carbon, known as the β-carbon, contains an acidic proton that, when subjected to nucleophilic attack under basic conditions, initiates a (β-)elimination reaction, yielding free glycans and unsaturated peptides. This β-elimination reaction successfully releases O-glycans.
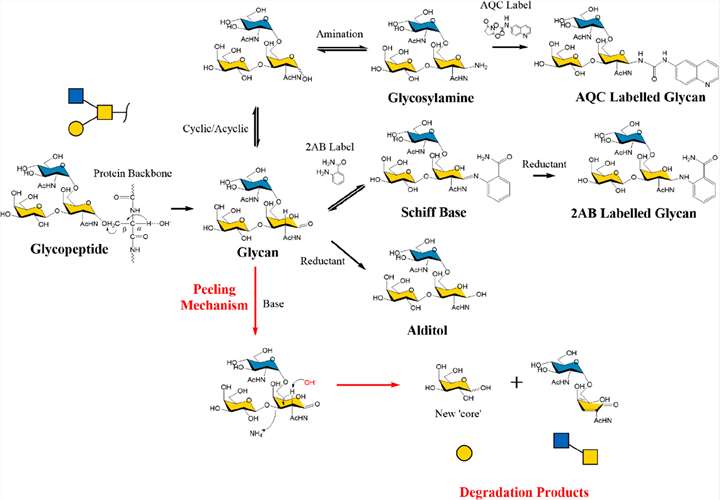 Fig. 1. O-Glycan
β-elimination, peeling mechanism, and end-capping strategies. (Wilkinson H, et al., 2020)
Fig. 1. O-Glycan
β-elimination, peeling mechanism, and end-capping strategies. (Wilkinson H, et al., 2020)
O-glycan modification and modification site analysis service is an effective and ingenious method to determine the O-glycan modification site.Creative Proteomics' lab technicians achive this goal by introducing a stabilizing substituent (an ether-linked methyl group) on each free hydroxyl group in natural glycans.
Throughout the service, glycosidic bonds, which are less stable than the ether-linked methyl group, are cleaved by acid hydrolysis after the introduction of the ether-linked methyl group, thus yielding a single methylated monosaccharide with a free hydroxyl group at the previously introduced linkage position. Then our scientists use a reducing agent (usually borohydride) to ring-open the partially methylated monosaccharides to introduce new hydroxyl groups, thereby identifying the reducing end of each monosaccharide.
Creative Proteomics tries to improve O-glycan modification and modification site analysis services in four aspects, including O-glycoprotein/peptide enrichment, O-glycan dissociation, O-glycan structure identification and quantitative analysis. You only need to tell us the purpose of your experiment and send your samples to us, we will take care of all the follow-up matters of the project.
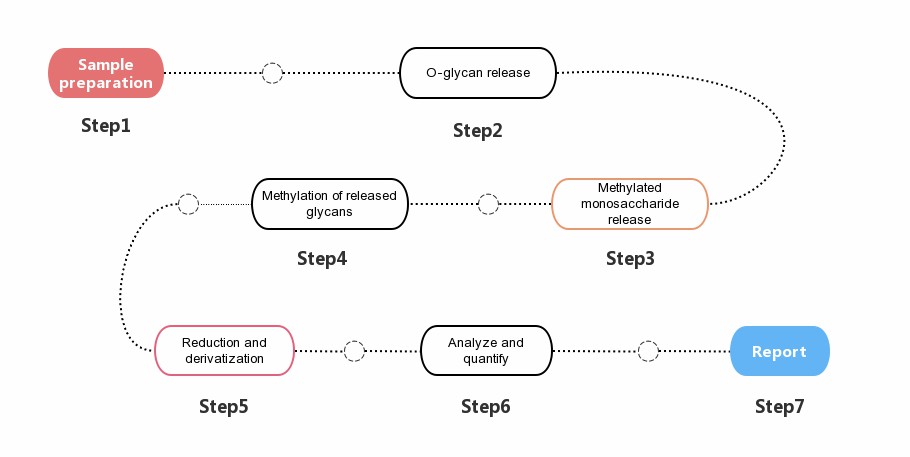 Fig. 2. General
workflow for O-glycan analysis.
Fig. 2. General
workflow for O-glycan analysis.
*Note: If you have any questions about sample delivery, please feel free to contact us, our experts are always on call.
The ICH Q6B guideline requires comprehensive characterization of glycoprotein glycosylation. With years of experience and an experienced scientific team, Creative Proteomics provides diverse and systematic O-glycan modification and modification site analysis services. Additionally, we can provide fully custom project designs to meet any specific requirements. If you are interested, please contact us or send us an inquiry directly.
References
For research use only, not intended for any clinical use.