Validation of Cell Line Expression Products
- Home
- Services
- Protein Sequencing
- Edman Based Protein Sequencing
- Validation of Cell Line Expression Products
Service Details
Stable cell lines are widely used for recombinant protein and antibody production, drug screening, gene function studies, and other applications. They can grow continuously for long periods of time and stably carry genetic modifications or express transgenes without significant changes in expression levels. There are two main ways to prepare recombinant proteins in industry: transient transfection and stable transfection. Transient transfection is often used to prepare small amounts of protein (up to the gram scale) for protein activity or animal testing early in drug development. Unlike transient transfection, stable cell lines are constructed for the industrial production of therapeutic proteins. Stable cell lines require the ability to produce the same quality product at different times, at different sites, and between batches. However, after several passages of cell lines, genetic instability may occur. Therefore, it is of great significance to verify whether the N-terminal methionine and signal peptide of the protein products are correctly processed during the establishment and fermentation of cell lines.
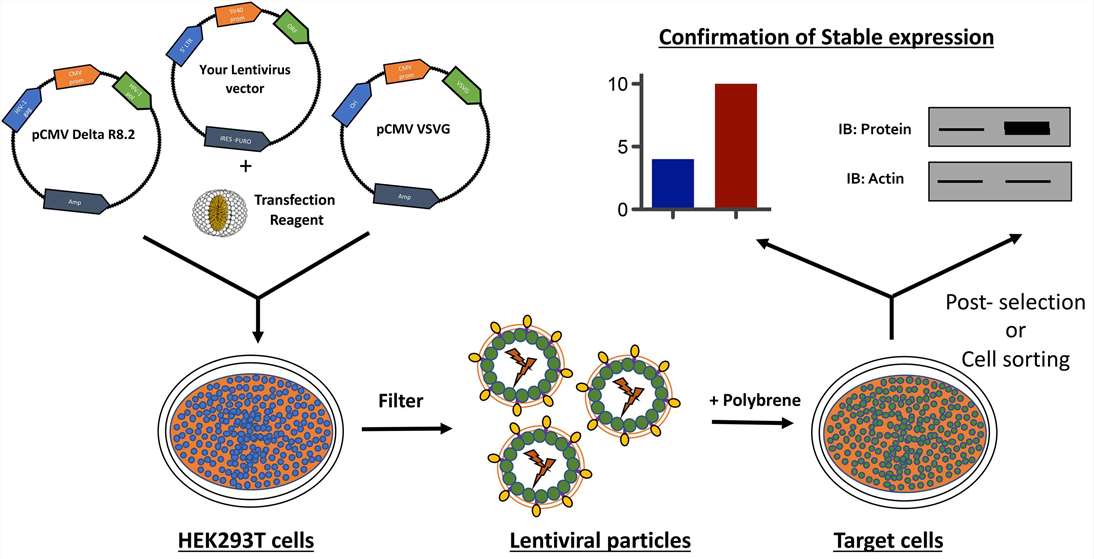 Fig. 1. Schematic
representation of generation of stable cell lines using lentivirus. (Tandon N, et al., 2018)
Fig. 1. Schematic
representation of generation of stable cell lines using lentivirus. (Tandon N, et al., 2018)
Creative Proteomics has been devoted to the research of recombinant protein expression and preparation for many years. The analysis process of the purified product of recombinant protein expression (cell line construction and fermentation) requires confirmation of the N-terminal sequence of the protein. Relying on the company's existing Edman sequencing system, Creative Proteomics' experienced technicians can provide high-quality protein N-terminal sequencing services for researchers and scientific research customers.
Edman degradation method that we provided can accurately determine protein N-terminal up to 60-70 amino acids Sequence, this method can label and cleave peptides from the N-terminus without breaking the peptide bonds between other amino acid residues, and accurately identify the N-terminal methionine and signal of protein products during cell line establishment and fermentation Whether the peptide is processed correctly.
Purified proteins (dissolved in or lyophilized from low-salt buffers) or SDS-PAGE bands blotted onto PVDF are suitable. A minimum of 25 pmol of protein (five steps) is required, more is strongly recommended for improved signal quality.
Creative Proteomic provides global customers with professional validation of cell line expression products services, which could be used to verify whether the N-terminal methionine and signal peptides of protein products are correctly processed during cell line establishment and fermentation. At Creative Proteomics, our highly qualified team works with our customers stand together on the front lines to help you solve tough research challenges. If you are interested in our services, please contact us immediately.
References
For research use only, not intended for any clinical use.