Biopharmaceutical Disulfide Bond Analysis Service
- Home
- Applications
- Biopharmaceutical Characterization Services
- Biopharmaceutical Variation Analysis Services
- Biopharmaceutical Disulfide Bond Analysis Service
Service Details
Disulfide bonds (SS bonds) are formed by the oxidation of sulfhydryl groups (-SH) on two cysteines in proteins, which is an important post-translational modification of protein therapeutics. Monoclonal antibodies (mAbs), the fastest growing class of biotherapeutics, have a wide range of therapeutic and diagnostic applications. The higher-order structure of mAbs plays a crucial role in efficacy and safety. For example, the number of disulfide bonds and their location are critical quality attributes (CQA) of mAbs, as incorrect disulfide bond formation can lead to loss of biological activity and even elicit an immune response in the host. Therefore, the confirmation of disulfide bonds plays a very important role in the process of confirming the structure of antibody drugs.
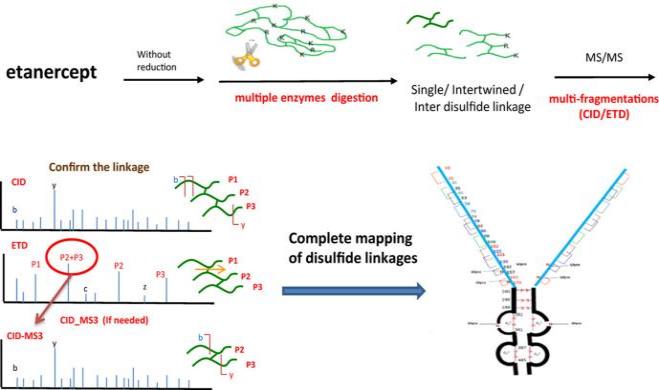 Fig.
1. Workflow for Analysis of Disulfide Bonds by Multienzyme Digestion Combined with LC-MS/MS. (Lam AK, et
al., 2022)
Fig.
1. Workflow for Analysis of Disulfide Bonds by Multienzyme Digestion Combined with LC-MS/MS. (Lam AK, et
al., 2022)
Disulfide linkage assignment, localization, and monitoring are important to ensure process consistency and product integrity during biotherapeutic drug development. In addition, regulatory agencies have specific requirements for mapping disulfide bonds in biotherapeutics such as monoclonal antibodies (mAbs). Here, Creative Proteomics introduces a comprehensive protein disulfide bond analysis solution to help you in your research, including normal, dense, and mismatch disulfide bond analysis, etc.
According to ICH Q6B guidelines, the biotherapeutics should determine the number and location of any free sulfhydryl and/or disulfide bonds whenever possible. Peptide mapping (under reducing and non-reducing conditions), mass spectrometry, or other appropriate techniques may be useful for this assessment. We introduce a highly sensitive HPLC-MS/MS platform for this purpose to analysis disulfide bonds in multiple samples from different sources.
Here, our expert team provides you with comprehensive disulfide bond analysis services, including analysis of unknown disulfide bonds, verification of disulfide bond mismatches in proteins, and disulfide bond mapping, etc.
Creative Proteomics provides one-stop disulfide bond analysis services, including sample preparation, protein purification, enzymatic digestion, LC-MS/MS identification and bioinformatics analysis. You just need to tell us your experiment purpose and send us your samples, we will take care of all follow-up matters of the project.
Creative Proteomics is a reliable biopharmaceutical partner. Our professional team can provide customers with comprehensive biopharmaceutical disulfide bond analysis service. Our services guarantee accurate and reliable results, at quick turnaround time! If you would like more information about specific aspects of our services, feel free to contact us and we will be happy to answer any questions.
References
For research use only, not intended for any clinical use.