Antibody N-Terminal Cyclization Analysis Service
- Home
- Applications
- Biopharmaceutical Characterization Services
- Biopharmaceutical Variation Analysis Services
- Antibody N-Terminal Cyclization Analysis Service
Service Details
The common variation at the N end is usually due to incomplete processing of the N- terminal signal sequence, resulting in the N- terminal methionine residue being not removed or the N- terminal Glu and Gln residues partially or completely forming pyroglutamic acid. Among them, N-terminal glutamic acid (Glu) can be cyclized to form pyroglutamic acid (pGlu), which is one of the main N-terminal modifications of mAbs. Although the effect of N-terminal cyclization on the potency of mAbs is unclear, it is important to understand the factors that govern pGlu formation during N-terminal cyclization in therapeutic mAb development due to most recombinant monoclonal antibodies (mAbs) contain glutamic acid and/or glutamine at their N-termini. In addition, N-terminal variants should be considered in risk assessment and characterization studies to rule out impact on product quality, safety, and efficacy to meet regulatory requirements.
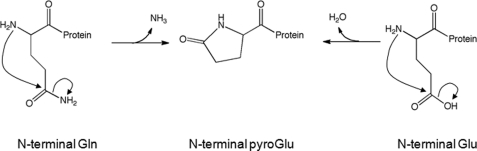 Fig. 1. Pyroglutamate formation
mechanism. (Liu Y D, et al., 2011)
Fig. 1. Pyroglutamate formation
mechanism. (Liu Y D, et al., 2011)
Monoclonal antibodies (mAbs) are complex glycoproteins that are usually produced using mammalian cells, resulting in complicated and heterogeneous post-translational modifications (PTMs). Among the various PTMs, N-terminal cyclization is one of the product quality attributes that may impact product quality, safety, and efficacy.
As a forward-looking company and a market leader in mass spectrometry, Creative Proteomics has successfully completed many challenging projects in antibody analysis. Our experienced scientists and dedicated analytical team will deploy optimal methods to help you perform N-terminal cyclization analysis and meet the requirements of every stage of drug discovery.
At Creative Proteomics, our professional team usually uses separated fractions by liquid chromatography-mass spectrometry (LC-MS) analysis for identification.
In addition to N-terminal glutamine cyclization analysis, with extensive expertise in PTM analysis, Creative Proteomics can apply orthogonal analysis methods to identify a series of PTMs, including glycosylation, C-terminal lysine variation, N-terminal Cyclization, deamidation, etc. We can meet the varying requirements of regulatory expectations and industry guidelines (ICH Q6B, PharmEu and USP<1047>).
Creative Proteomics is a reliable biopharmaceutical partner. Our professional team can provide antibody N-terminal cyclization analysis service for global customers. We will provide clear, comprehensive written reports, recommendations and agreements, as well as customized services to help clients solve analytical and technical problems. If you would like more information on a specific aspect of our services, please do not hesitate to contact us, we will be happy to answer any questions.
References
For research use only, not intended for any clinical use.