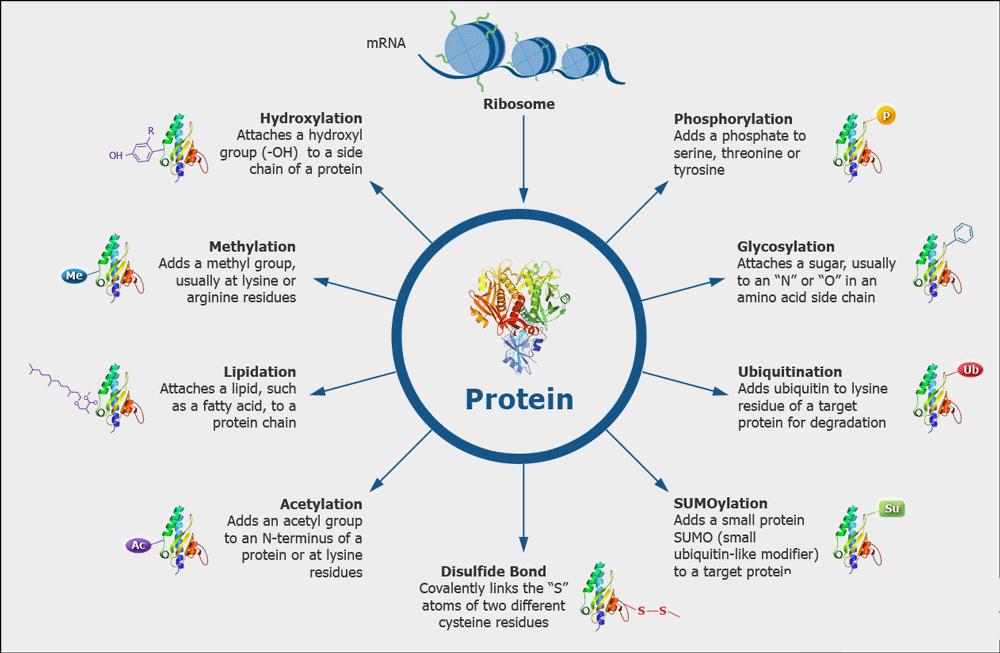
Protein Characterization All in One Place - Your Ultimate Guide to Comprehensive Solutions
Looking to streamline the development of your protein therapeutics? This ultimate solution guide will walk you through everything you need to know about protein characterization, ensuring your products meet the highest standards of safety, efficacy, and regulatory compliance.
Get an Instant Quote NowIn line with the ICH Q6B Guidance
Jump to Section
- Protein Drug Characterization
- Antibody Drug Characterization
- Types of Therapeutics
- Instrument Platform
- Characterization Process
- Service Advantages
- FAQs
Why Characterize Therapeutic Protein?
From technical guidelines perspective
Recombinant proteins, a major class of therapeutic proteins, are increasingly favored over small molecules like RNAs, oligonucleotides, peptides, or vaccines due to their cell-specific actions and lower toxicity. As the development of protein therapeutics continues to grow rapidly, it is expected to match or even surpass traditional small molecule drug development.
To ensure the consistency, quality, and purity of protein therapeutics, stringent monitoring of the manufacturing process and the final product is essential. Accurate and reproducible characterization methods are crucial in guiding decisions related to the manufacturing process and product formulation. A well-characterized biologic ensures safety and efficacy by accurately defining identity, heterogeneity, impurity, and potency. Given the complexity of proteins, multiple analytical methods are required to meet these standards, as no single platform can address all characterization needs. In-depth characterization is necessary for therapeutic proteins to gain approval for clinical trials and market release.
Key points:
- Batch-to-batch consistency: Guarantees safe delivery throughout the entire process.
- Quality Control (QC): Ensures drug identity, strength, potency, purity, and safety.
The current quality standards of protein therapeutics mainly refer to the relevant technical guidelines of International Conference on Harmonization of Registration Pharmaceuticals for Human Use (ICH), Food and Drug Administration (FDA), United States Pharmacopoeia (USP) and European Pharmacopoeia (EP).
| Inspection Category | Items Included |
|---|---|
| General Inspection | Appearance, color, turbidity, visible foreign matter |
| Physicochemical Inspection | Molecular weight or size, extinction coefficient, Electrophoretic patterns |
| Purity and Impurity Analysis | Protein purity determination, molecular size variants, charge variants, product-related and process-related impurities |
| Content Determination | Protein content determination |
| Identification and Consistency Analysis | Amino acid sequence, amino acid composition, terminal amino acid sequence, molecular structure |
| Potency Analysis | Biological activity, binding activity |
Protein Drug Characterization
| Characterization Service | Research Project | Method |
|---|---|---|
| Protein Identification | Molecular Weight | ESI-MS |
| MALDI-TOF-MS | ||
| Primary Sequence | Peptide Mapping | |
| N-terminal sequencing by Edman degradation | ||
| N- or C-terminal sequencing with MALDI-ISD | ||
| Isoelectric Point | Isoelectric Focusing | |
| Capillary Isoelectric Focusing | ||
| Protein Homogeneity Analysis |
Protein Aggregation Analysis | SEC |
| Protein Purity Analysis | HPLC | |
| Protein Fragment Analysis | HPLC | |
| Protein Isoform Analysis | Capillary Isoelectric Focusing | |
| Host Cell Proteins Analysis | ELISA, 2D western blog, LC-MS/MS | |
| Host Cell Residual DNA analysis | qPCR/dd PCR | |
| Glycosylation Analysis | Glycan Analysis | MALDI-TOF |
| Glycopeptides Analysis | LC-MS/MS | |
| Other Protein PTMs Analysis | Disulfide Bond, Phosphorylation, etc. | LC-MS/MS |
| Advanced Structure Analysis | Secondary Structure Analysis | Circular Dichroism Spectra (Far UV) |
| Fluorescence Spectroscopy | ||
| Nuclear Magnetic Resonance (NMR) spectroscopy | ||
| FT-infrared Spectroscopy | ||
| Tertiary Structure Analysis | NMR Spectroscopy | |
| HDX-MS |

Detailed Protein Characterization Guide
Get a complete overview of the essential methods and techniques for protein and antibody characterization. From molecular weight determination to advanced structure analysis, this guide provides the key insights to ensure the quality of your biopharmaceuticals.
Antibody Drug Characterization
Our essential guidelines for antibody drug characterization include key considerations to ensure accurate analysis, improve product consistency, and meet regulatory standards.
Visit our characterization services page for more details.
| Characterization | Research Project | Method |
|---|---|---|
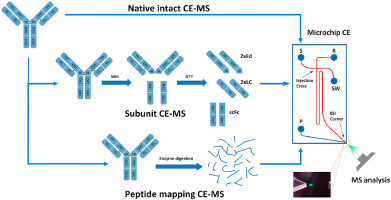
| Molecular Weight | Intact molecular weight | ESI-Orbitrap MS |
| Light and heavy chain molecular weight | ESI-Orbitrap MS / MALDI-TOF MS | |
| Fab fragment and Fe fragment molecular weight | ESI-Orbitrap MS / MALDI-TOF MS | |
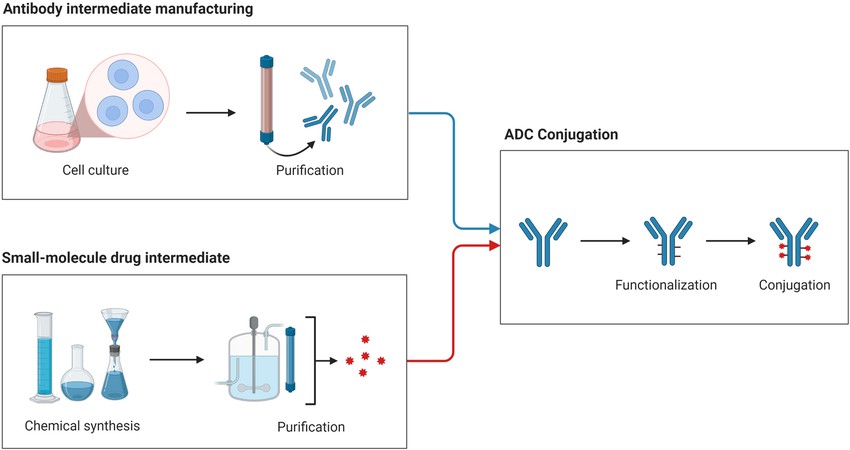
| ADC molecular weight and DAR value analysis | HPLC-MS | |
| Protein Homogeneity Analysis |
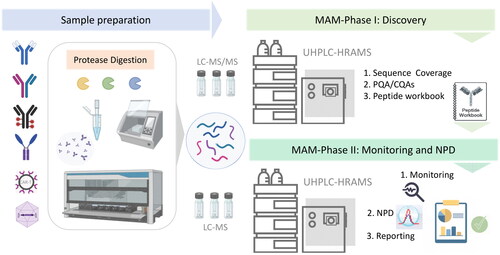
Peptide Mapping (protein amino acid sequence confirmation) | LC-MS/MS |
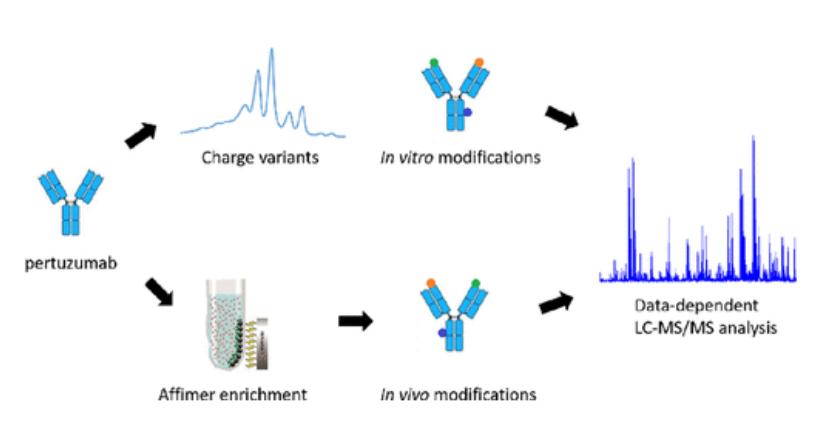
| Other Analysis | Charge heterogeneity analysis | IEF / CE-IEF / cIEF |
| Protein aggregation analysis | SEC / GPC | |
| Modification Analysis | Disulfide bond analysis | LC-MS/MS |
| Glycosylation analysis | MALDI-TOF / LC-MS/MS | |
| Glycan analysis | ||
| Glycopeptides analysis | ||
| Phosphorylation analysis | LC-MS/MS | |
| Deamidation and oxidation analysis | LC-MS/MS | |
| Purity Analysis | Monomer and polymer purity analysis | SEC-HPLC |
| Non-glycosylated heavy chain and small molecule fragments | SEC-HPLC / SDS-PAGE / CE-SDS | |
| Binding Assay | Protein binding/inhibition analysis | ELISA (EC50, IC50) |
| Cell binding/inhibition analysis | FACS (EC50, IC50) | |
| Impurity Detection and Analysis | Host Cell Proteins Analysis | 2D-DIGE / LC-MS/MS |
| Host Cell Residual DNA analysis | Real-time PCR |

What Types of Therapeutics Can We Characterize for You?
We offer a range of customizable biopharmaceuticals characterization services tailored to your specific needs. Our services encompass a variety of sample types, including but not limited to:
Instrument Platform
Creative Proteomics is equipped with a comprehensive suite of advanced analytical technologies, including NMR, HDX, ion mobility mass spectrometry, and various other mass spectrometry techniques. These tools enable detailed characterization of the advanced structural features associated with key quality attributes of protein therapeutics. Additionally, our capabilities extend to the use of TEM, XRD, and SEC-MALS for the identification and characterization of protein drug aggregates.
Service Advantages
Efficient Turnaround
Based on the specific project requirements, we will establish a reasonable timeline to ensure efficient testing and timely reporting. This approach saves time and costs, thereby enhancing research and development efficiency.
Sophisticated Instruments
We have multiple high-end instruments, such as the AB Sciex 6500 series and Thermo Fisher Q Exactive. These advanced devices ensure high accuracy and reliability in our testing, enabling us to handle a wide range of challenging analytical projects.
Comprehensive Service
We offer a variety of assay platforms ranging from omics to biopharmaceutical characterization and pharmacokinetics, enabling comprehensive assay analysis from omics to single molecule. This wide range of platforms allows us to meet various complex analytical needs.
Rich Experience
Our technical team has successfully assisted numerous clients in analyzing related drugs and provided high-quality professional services.
Streamlined Protein & Antibody Characterization Process
- 1
Project Kickoff & Consultation
We start by understanding your specific project needs through a detailed consultation with our experienced scientists. This ensures we align our services with your objectives and regulatory requirements.
- 2
Customized Service Proposal
Based on your needs, we provide a detailed proposal outlining the scope of work, methodologies, timelines, and costs, tailored to your specific drug characterization goals.
- 3
Sample Management
We guide you through the sample preparation and shipment process, providing clear instructions to ensure safe and compliant handling. Once received, we confirm sample integrity before proceeding.
- 4
In-Depth Analysis & Quality Control
Our team conducts precise and thorough analysis using state-of-the-art techniques and instrumentation. Rigorous quality control measures are applied at every stage to guarantee reliable and accurate results.
- 5
Results & Reporting
Upon completion, we deliver a comprehensive report that includes detailed findings, expert analysis, and actionable insights. We ensure the report meets your expectations and regulatory needs before finalization.
- 6
Ongoing Expertise & Support
We remain committed to your success even after the project concludes, providing continued support for any further analysis or adjustments needed as your project evolves.
FAQs
What is the amount of protein required for intact mass determination?
40-50 μg of proteins will be enough for us to perform protein mass determination by MALDI-TOF or ESI-Q-TOF.
What is the amount of protein required for protein identification?
5-10 μg of proteins will be enough for us to get the consistent results by using our nanoLC-MS/MS.
Can you perform protein quantification analysis?
Yes, we provide protein quantification analysis by using various method including SlLAC, TRAQ/TMT and label-free.
How should l prepared samples?
As for the sample preparation, you may provide us the proteins in gels, in solution or lyophilized samples. If you want to provide us the samples in solution and follow up Ms step, we would like to suggest that the buffer should be in low salt concentration and without any detergents.
How should l send my samples?
As for the shipment, you may send us the lyophilized powder or gel sample in ice pack or the solution sample in dry ice.
Ready to Get a Protein Characterization Quote?
Get Your Instant QuoteRely on our expert team to craft tailored characterization solutions, providing you with precise analytical insights that drive confident and informed decisions throughout your development journey.