The special N-glycosylation structure is related to the safety and effectiveness of many protein drugs. Determining the exact N-glycan site occupancy can be used to comprehensively analyze the glycosylation of proteins, therefore, the measurement and analysis of N-glycan sites is an important biochemical technology for biopharmaceutical development projects. The technical method can be used to determine the type and content of N-glycosylation sites of biopharmaceutical proteins or other biological agents, determine the structure and relative content of glycosylation, and also measure free N-glycans.
Creative Proteomics is a reliable biopharmaceutical partner with professional glycobiology research resources. We use advanced equipment and instruments and powerful operability analysis methods to provide you with high-quality one-stop service. N-glycan site analysis is an important part of ICH Q6B specification. We will provide you with comprehensive and GLP / cGMP-compliant N-glycan site technical analysis services around the ICH guidelines (especially ICH Q6B) and the US FDA issues 'Points to Consider' document.
The cartoon representation of six glycoproteins (PDB id + chain id) used for MD simulation study (Lee H S et al. 2015.)
We Can Provide but Not Limited to:
- N-glycosylation site analysis
- N-glycan structure analysis
- Relative content of N-glycan analysis
- Releasedglycan analysis
- Sequence analysis of peptides modified by N-glycosylation site
- Glycoprotein full molecular analysis
- Identify atypical N-glycosylation sites present in protein / peptide drugs
Technology Platform of N-glycan Sites Occupation Analysis Service:
Creative Proteomics provides effective and accurate analysis of N-glycanylation sites through N-glycan release technology, N-glycan derivatization, and HILIC high-performance liquid chromatography, C18-RP-LC-ESI-MS technology analysis, high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD) and high-performance liquid chromatography fluorescence detection (HPLC-FLD), etc.
We provide the following analysis methods:
1) Derivatize the released glycans by ethyl esterification to esterify α2,6-linked or α2,3-linked ethyl esters, and detect the connection-specific sialic acid, using purified HILIC for purification Methods Purify the glycans and analyze the N-glycosylation site information using C18-RP-LC-ESI-MS technology. The specific workflow is as follows.
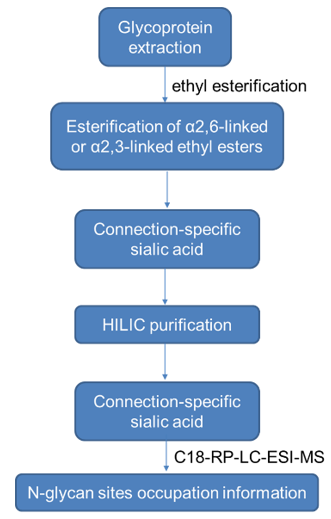 The workflow of N-glycan sites occupation analysis technology.
The workflow of N-glycan sites occupation analysis technology.2) The glycoprotein was first digested with trypsin, then the sugar chain linked to ASN was excised using PNGase F glycosidase, and then glycopeptide was separated using HPLC-FLD and high pH HPAE-PAD. Analyze and quantify labeled ortho-glycans, detect deglycosylated peptides, and use MS to confirm the sequence of glycosylated modified peptides and N-glycosylation site information of glycopeptides. The specific workflow is as follows.
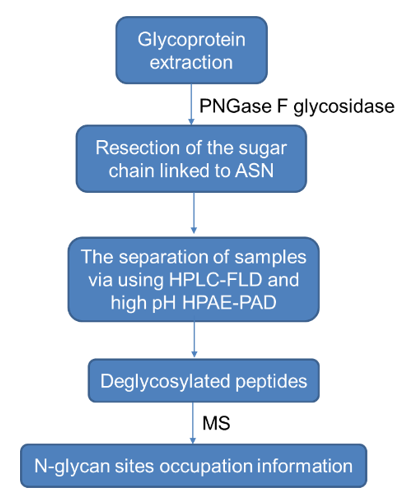 The workflow of N-glycan sites occupation analysis technology.
The workflow of N-glycan sites occupation analysis technology.Advantages of N-glycan Sites Occupation Analysis Service:
- Short time-consuming: The equipment used in this technology is highly automated, the sample preparation steps are simple and easy, and the analysis time is greatly reduced.
- High sensitivity: Combined with fluorescent labeling and mass spectrometry analysis methods, high sensitivity can be achieved during the analysis of N-glycosylation sites.
- High resolution: The core of the N-glycan site analysis workflow is the HILIC column specially designed for macromolecular separation, which can achieve higher resolution when separating macromolecular free N-glycan.
- High-throughput: The service technology has a robot-based high-throughput glycosylation analysis platform that can analyze multiple glycoprotein samples simultaneously.
- Rapid turnaround time: 5-7 days to provide detailed technical reports.
- Customized service: We can customize professional solutions for you according to your research plan needs. You can select or suggest the required items for analysis.
Creative Proteomics's professional researchers can provide our customers with complete confirmation and analysis of N-glycan sites in biopharmaceutical proteins. We will provide you with detailed experimental process steps, related peptide map analysis and final N-glycosylation site analysis report and other data reports. We are committed to providing you with expertise in extended neighborhood analysis to help you solve problems related to analysis and technology.
References
- PaHJnsn. Structural analysis of N- and O-glycans released from glycoproteins. Nature Protocols, 2012.
- Robbe C, Capon C, et al. Microscale analysis of mucin-typeO-glycans by a coordinated fluorophore-assisted carbohydrate electrophoresis and mass spectrometry approach. Electrophoresis, 2010, 24(4):611-621.
- Mereiter Stefan, Gomes Joana, et al. Glycomic Approaches for the Discovery of Targets in Gastrointestinal Cancer. Frontiers in Oncology, 2016.






