As the fastest growing sectors of the pharmaceutical industry, most therapeutic proteins have complex post-translational modifications for efficient secretion and drug efficacy and stability maintenance. PTMs can affect the activity, stability, and function of a protein. Misfolding, aggregation, variable glycosylation, oxidation of methionine, deamination of asparagine and glutamine, and proteolysis are common types of protein modifications. Incorrect modifications of protein therapeutic may result in immune response, or even serious side effects. These modifications pose a great challenge for accurate and consistent bioprocessing and a comprehensive analysis of protein modifications is a necessary.
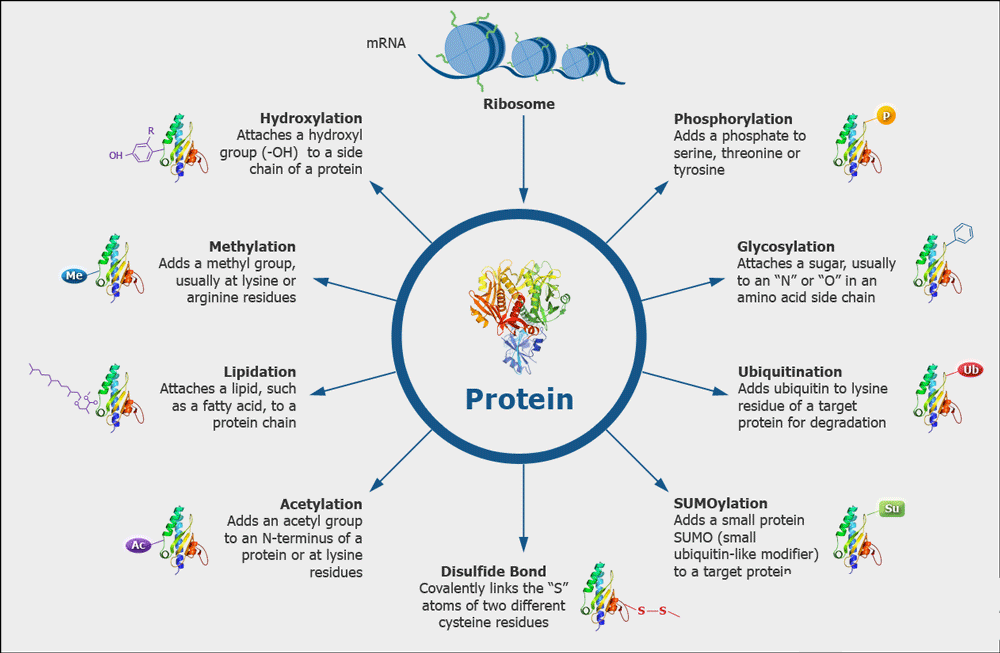
Fig1. Various Kinds of Protein Modifications
Radioactive labeling and Western blotting are the two traditional strategies for protein modifications analysis, though specific and relatively quantitative, depending on prior knowledge of the modification type and the availability and specificity of the antibody. The new Pro-Q diamond staining provides a convenient method for the evaluation of global protein phosphorylation, yet still not enough for fully characterization of the protein because neither the proteins nor their modification sites can be identified. In contrast, the mass spectrometry (MS) can not only identify the protein, the modification types, but also locating the modifications sites without previous knowledge. Therefore, MS is widely used for protein modifications in biopharmaceutical industry for quality control and lot release.
Bottom-up and top-down are the two complementary approaches in MS-based proteomics. Bottom-up shotgun proteomics involves proteolytic digestion of proteins with enzymes into peptides fragments before MS analysis. With high throughput and automation, this approach is appropriate for the protein identification from the database with limited sequence coverage. Even if all all modifications present in the digested peptides are identified, the modification status of the sequence not covered remains unknown. Therefore, this approach has its intrinsic limitations for fully protein modifications characterization. In contrast, top-down approach is a powerful technology for characterization of protein modifications. The labile structure destroyed in bottom-up is preserved in this strategy. Without prior knowledge, all the existing modifications including PTMs such as phosphorylation, methylation and acetylation and sequence variants included mutants, various isoforms can be identified simultaneously in one spectrum. Coupled with electron capture dissociation (ECD), top-down is successfully used for the multiple modifications identification, complete modifications mapping with full sequence coverage, unexpected PTMs and amino acid polymorphisms discovering. However, the top-down proteomics is still in its early stage, compared to well-established bottom-up approach, because of technical challenges in sample preparation, instrument sensitivity and throughput.
Protein Modifications Analysis at Creative Proteomics are as below.
| Glycosylation Analysis | N-glycans Profiling |
| O-Glycans Profiling | |
| N-glycan Sites Occupation Analysis | |
| O-Glycans Sites Occupation Analysis | |
| Glycopeptides Analysis | |
| Siliac Acid Analysis | |
For more details, please feel free to contact us or send inquiry directly.






