Protein N-myristoylation is a universal post-translational modification in eukaryotes catalyzed by the enzyme N-myristoyltransferase (NMT), which transfers myristate from myristoyl coenzyme A (Myr-CoA) to the N-terminal glycine of a wide range of substrate proteins, it has been involved in the progression and development of a range of human diseases, such as cancer1, epilepsy, Alzheimer’s disease, Noonan-like syndrome and viral and bacterial infections. Both NMT1 and NMT2 of human NMT isozymes are conveyed in most tissues. N-myristoylation is certainly surpassed by proteolysis to show an N-terminal glycine, the only completely conserved motif across all known NMT substrates. This may happen either co-translationally when methionine aminopeptidase (MetAP) acts on an N-terminal ‘MG’ motif at the ribosome, or post-translationally at an internal site. Besides the importance in health and disease of N-myristoylation, direct identification of the N-myristoylated proteome in cells has been limited to non-native low-throughput systems, for example, over-expression of artificial protein constructs. N-terminally myristoylated proteins are related to many kinds of cellular processes, which includes proliferation, differentiation, and apoptosis, and have been shown to be essential for embryogenesis.
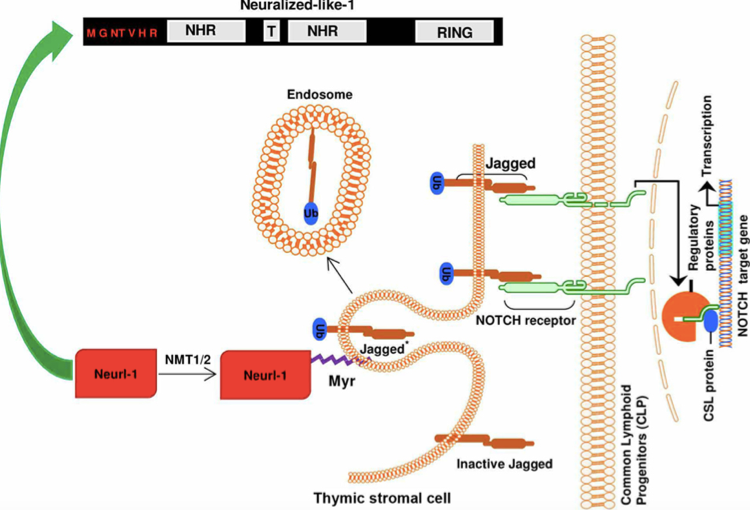 Figure 1: N-myristoylation of neutralized like 1 (neur1) during notch signaling
Figure 1: N-myristoylation of neutralized like 1 (neur1) during notch signalingN-myristoylation is an irreversible protein modification that involves the covalent attachment of N-tetradecanoic acid (myristic acid) to the N-terminal glycine residue of proteins. Myristoyl-CoA of protein brings its activity into play after cleavage of the initiator methionine residue or after exposure of an internal glycine. Several mammalian and plant species were showed to express two isoenzymes of NMT (NMT1 and NMT2) localized in the cytosol. NMT1 and NMT2 share a high specificity for myristoyl-CoA, even though they have different peptide substrate specificities. In fatty acid chain length, an increase or decrease significantly reduces the incorporation rate of the fatty acid into the peptide. The ADP-ribosylation factors (Arfs), a family of small GTP-binding proteins of the Ras superfamily, is the most extensively characterized N-myristoylated proteins in yeast and mammals, which involved in intracellular protein transport and secretion.
Creative Proteomics has a strict workflow to analyze N-myristoylation to meet your requirements.






