- Why DSC Matters
- Services
- FAQ
- Demo
- Case Study
- Related Services
- Support Documents
- Inquiry
1. Introduction to Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimetry (DSC) is a cornerstone biophysical technique used to investigate the thermal behavior of proteins, antibodies, and other biological macromolecules. By precisely measuring the heat flow associated with molecular transitions, DSC provides direct insight into protein folding, stability, and conformational integrity—critical parameters in biomedical research and pharmaceutical development.
In contrast to other analytical methods that infer structural changes indirectly, DSC quantifies the thermal stability of biomolecules through their heat capacity changes as they are gradually heated. This makes it an invaluable tool for scientists who need accurate, label-free, and reproducible data on protein behavior under thermal stress.
Whether you're assessing formulation stability, comparing biosimilar candidates, or optimizing biopharmaceuticals for storage and delivery, Differential Scanning Calorimetry offers a high-resolution view of thermal transitions that directly impact molecular performance and integrity.
Why DSC Matters in Biomedical and Pharmaceutical Research
Differential Scanning Calorimetry analysis plays a pivotal role in the development, formulation, and quality assessment of biopharmaceuticals. By revealing how proteins respond to temperature-induced stress, DSC offers critical insight into their structural integrity—an essential parameter for ensuring product consistency and therapeutic reliability in research contexts.
Protein Thermal Stability and Folding Assessment
In biomedical research, protein thermal stability is a proxy for proper folding and conformational resilience. DSC enables precise determination of melting temperature (Tm), which marks the point at which a protein unfolds. A higher Tm typically indicates greater structural stability—a key consideration during protein engineering, storage optimization, and stress testing.
Formulation Screening and Optimization
Formulation scientists use DSC to evaluate the stabilizing effects of excipients, pH conditions, and buffer systems. A shift in the Tm under different conditions can indicate whether a formulation promotes or compromises protein stability. This makes DSC indispensable during preclinical formulation development, where optimizing shelf life and structural robustness is a high priority.
Biosimilar Characterization and Comparability
One of the most impactful applications of Differential Scanning Calorimetry analysis is in the comparability assessment of biosimilars. Regulatory expectations require that biosimilars closely match their reference products in structural and functional attributes. DSC thermograms provide fingerprint-like profiles that allow for side-by-side comparison of thermal unfolding behaviors, revealing even subtle differences in protein domains.
Accelerating Biopharmaceutical R&D
For pharmaceutical R&D teams, DSC is often integrated early in the development pipeline to de-risk candidate selection. Proteins that exhibit low thermal stability may be deprioritized before entering more resource-intensive studies. By identifying thermally robust candidates early on, DSC contributes to faster, more cost-effective development workflows.
Our Differential Scanning Calorimetry Service: Capabilities and Deliverables
At Creative Proteomics, our Differential Scanning Calorimetry Service is tailored to support protein and antibody characterization across all stages of non-clinical research and biopharmaceutical development. Whether you are optimizing formulations, conducting comparability studies, or verifying batch consistency, our DSC services deliver high-quality, publication-ready thermodynamic data.
Instrument Platforms
We operate advanced, high-sensitivity calorimetry systems, including:
MicroCal™ PEAQ-DSC
Ideal for high-resolution analysis of protein unfolding transitions, even at low concentrations. Offers precise baseline stability and automated sample handling for high throughput.
Nano DSC (TA Instruments)
Suitable for small-volume, low-concentration samples. Enables accurate detection of subtle thermal transitions in sensitive biologics.
Sample Requirements
To ensure optimal results, please prepare samples according to the following general guidelines:
- Volume: Typically 300–500 µL per run
- Concentration: 0.5–2.0 mg/mL (may vary by protein type)
- Buffer Matching: Sample and reference buffers must be identical to minimize baseline artifacts
What You Receive
Every DSC analysis includes a comprehensive data package:
- Raw Thermograms in standard output formats
- Processed Data with baseline correction and integration
- Key Parameters: Tm, ΔH, ΔCp, and thermogram interpretation
- Summary Report: Detailed analysis, figures, and expert commentary
Why Choose Creative Proteomics?
- Over a decade of experience in protein and antibody characterization
- Expert scientific consultation with every project
- Fast turnaround and transparent project communication
- Data delivered with integrity and full interpretive support
Workflow of Differential Scanning Calorimetry Service
How Differential Scanning Calorimetry Works: Principles and Outputs
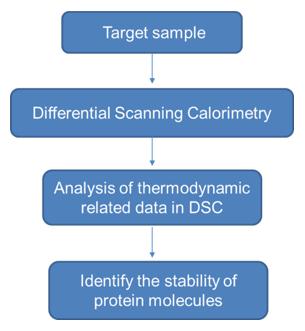
Principle of Operation
DSC works by comparing the heat flow into a sample cell containing the protein solution with that into a reference cell (typically containing buffer alone). As the temperature increases at a constant rate, any endothermic or exothermic transitions in the protein—such as unfolding or aggregation—are detected as deviations in heat flow between the two cells.
These differences are plotted as a thermogram, a curve showing heat capacity (Cp) as a function of temperature. Key events, such as the melting of protein domains, appear as distinct peaks on this curve.
Key Parameters Derived from DSC Thermograms
- Tm (Melting Temperature): The temperature at which 50% of the protein population is unfolded. A higher Tm suggests greater thermal stability.
- ΔH (Enthalpy Change): The total energy required for unfolding, calculated from the area under the peak. This reflects the cooperativity and strength of intramolecular interactions.
- ΔCp (Change in Heat Capacity): Provides insight into solvent exposure of hydrophobic regions and structural rearrangements.
Why It Matters
Unlike spectroscopic or chromatography-based methods that infer structural changes indirectly, DSC provides a direct and label-free assessment of thermal transitions. It does not require fluorescent tags or denaturants, preserving the native structure of the protein throughout analysis. This makes DSC uniquely suited for characterizing native-state stability and evaluating subtle conformational differences between formulations or product lots.
Key Applications of DSC in Protein and Antibody Characterization
DSC is a versatile technique with wide-ranging applications in protein science and biopharmaceutical research. By providing high-resolution thermodynamic profiles, DSC supports critical decisions throughout the drug development and manufacturing lifecycle—from candidate screening to quality control.
Protein Formulation and Stability Studies
One of the most common uses of DSC is in evaluating the thermal stability of proteins under various formulation conditions. By measuring shifts in Tm and enthalpy (ΔH) across different buffer compositions, pH levels, and excipient combinations, researchers can identify optimal conditions that enhance stability and minimize degradation during storage or transport.
This data is especially valuable in early formulation screening, where small-scale experiments guide the selection of lead formulations for downstream development.
Antibody Thermal Profiling
Monoclonal antibodies (mAbs) typically exhibit multiple unfolding transitions corresponding to their structural domains (e.g., Fab and Fc regions). DSC resolves these transitions with high sensitivity, enabling researchers to assess domain-level stability—a crucial factor in therapeutic antibody development.
Differential scanning calorimetry analysis has also been used to identify conformational differences caused by glycosylation variants, CDR mutations, or stress-induced modifications.
Comparability Studies for Biosimilars
In the biosimilar development pipeline, demonstrating structural and functional equivalence to a reference product is a regulatory requirement. DSC plays a key role in these comparability assessments by providing quantitative, reproducible thermodynamic fingerprints.
A close match in thermal profiles between originator and biosimilar candidates—especially in Tm and peak shape—supports higher-order structure similarity, complementing techniques like circular dichroism (CD) and mass spectrometry.
Quality Control in Biomanufacturing
DSC is also deployed in quality assurance workflows during manufacturing. Batch-to-batch consistency in thermal profiles is an indicator of product uniformity and stability. Deviations may point to issues such as formulation drift, degradation, or contamination—making DSC a valuable orthogonal tool for lot release and stability monitoring.
Integration with Other Analytical Services
While Differential Scanning Calorimetry (DSC) delivers powerful thermodynamic insights, its greatest value often emerges when integrated with complementary analytical methods. By combining DSC with orthogonal techniques, researchers gain a more complete understanding of a protein's structural integrity, heterogeneity, and formulation behavior.
Protein Sequence Analysis
Sequence fidelity is the foundation of accurate structural and functional characterization. Before performing DSC, we recommend confirming protein identity and purity using mass spectrometry-based sequence validation. This ensures that any thermal transitions observed are due to true protein behavior—not artifacts from degradation or contaminants.
Mass Spectrometry Characterization
Mass spectrometry (MS) provides detailed molecular information on post-translational modifications, glycosylation patterns, and sequence variants. When used alongside DSC, MS helps contextualize stability changes. For example, differential glycoforms or oxidation states may explain shifts in Tm or altered unfolding behavior.
Aggregation and Charge Variant Analysis
DSC reveals thermal transitions, but it does not directly detect aggregation or charge heterogeneity. To complete the picture:
Dynamic Light Scattering (DLS) or Size-Exclusion Chromatography (SEC) can assess aggregation state before or after DSC analysis.
Isoelectric Focusing (IEF) or Capillary Electrophoresis (CE) identifies charge variants that may influence protein conformation and thermal stability.
A Systems-Level Approach to Protein Stability
Integrating DSC with sequence analysis, MS profiling, and aggregation assays allows researchers to:
- Validate unfolding mechanisms
- Pinpoint the cause of destabilization
- Build a more predictive model for formulation and comparability studies
This multi-dimensional strategy is particularly valuable in biosimilar development, formulation screening, and quality control settings, where single-method approaches may fall short.
FAQs: Differential Scanning Calorimetry Services
Q: What are the typical sample requirements for DSC analysis?
A: For most protein samples, we recommend:
Volume: 300–500 µL
Concentration: 0.5–2.0 mg/mL
Buffer: Matched sample and reference buffer, ideally without detergents or reducing agents
If your sample deviates from these guidelines, our team can advise on feasibility or assist with buffer exchange.
Q: Can Differential Scanning Calorimetry detect protein aggregation?
A: While DSC is not a direct aggregation assay, it can reveal signs of aggregation through unusual thermogram shapes or shifts in enthalpy. For precise aggregation analysis, we recommend combining DSC with Dynamic Light Scattering (DLS) or Size-Exclusion Chromatography (SEC).
Q: How does DSC compare to ITC or DLS in protein analysis?
A: DSC measures heat capacity changes to assess thermal stability and protein unfolding.
ITC (Isothermal Titration Calorimetry) is used to study binding thermodynamics (e.g., ligand-protein interactions).
DLS measures hydrodynamic size and aggregation in solution.
These methods are complementary, not interchangeable, and are often used together in biophysical profiling workflows.
Q: What types of biomolecules can be analyzed using DSC?
A: DSC is commonly applied to:
- Monoclonal antibodies
- Enzymes
- Recombinant proteins
- Vaccine antigens
- Nucleic acid-protein complexes (in select cases)
If you're unsure whether your sample is suitable, our team is happy to provide a free technical consultation.
Q: How long does a typical DSC project take?
A: The duration of a DSC project varies based on factors such as sample complexity, the number of conditions tested, and analysis requirements. We prioritize efficient workflows and clear communication to ensure timely delivery of high-quality results. Expedited processing options are also available to meet urgent research needs.
Q: Can DSC be used in regulatory filings for biosimilars or biologics?
A: Yes—DSC is widely accepted as an orthogonal method for demonstrating higher-order structural similarity in biosimilar comparability studies.
Demo
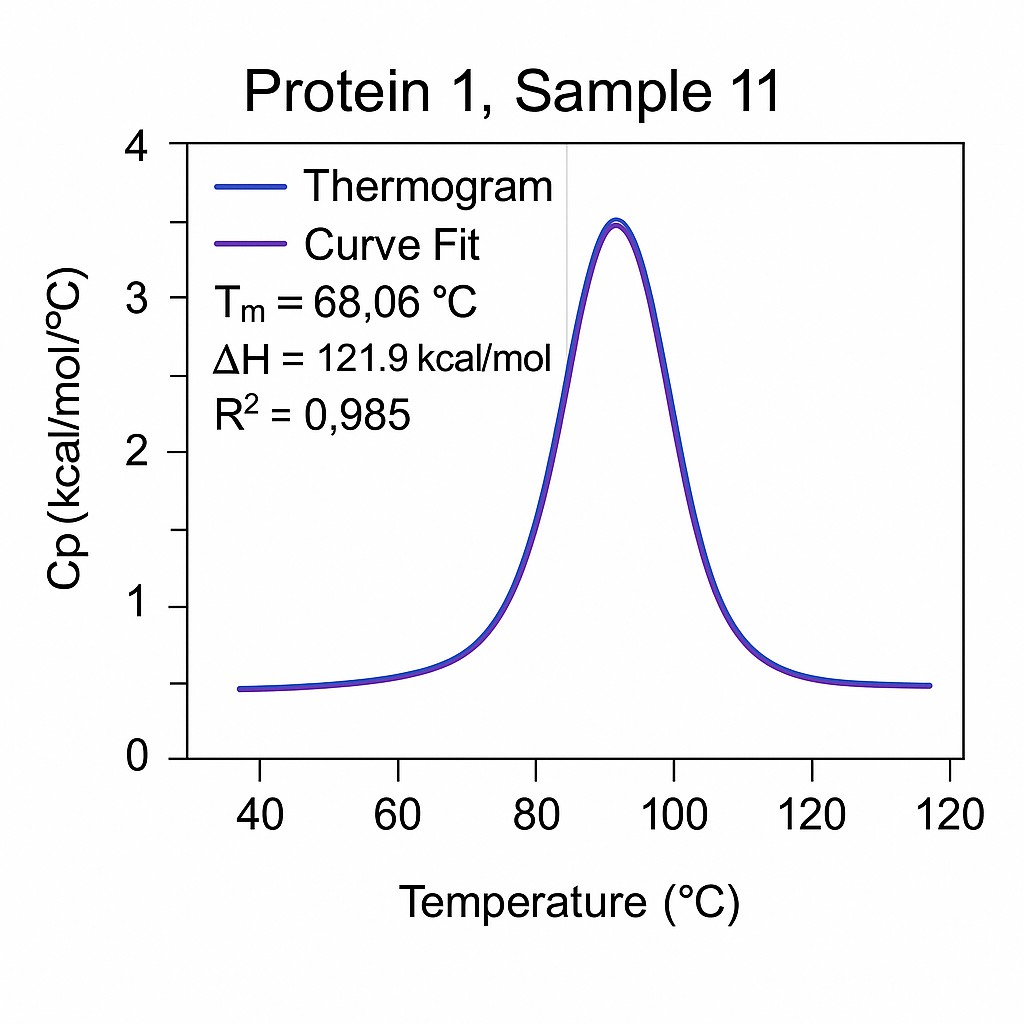
Case Study: Utilizing DSC to Evaluate Protein Thermal Stability
DOI: 10.3791/55262
Objective
The study aimed to demonstrate the application of DSC in evaluating the thermal stability and conformational integrity of protein antigens, which is crucial in biopharmaceutical development and quality control.
Methodology
Sample Preparation: Protein samples were prepared in appropriate buffer solutions.
DSC Analysis: Samples were subjected to DSC to measure heat flow associated with thermal transitions, providing thermograms indicative of protein unfolding events.
Data Interpretation: Key thermodynamic parameters, including melting temperature (Tm) and enthalpy change (ΔH), were derived from the thermograms to assess protein stabiylit.
Findings
Thermal Transitions: DSC thermograms revealed distinct endothermic peaks corresponding to protein unfolding, allowing determination of Tm values.
Stability Assessment: Comparative analysis of Tm and ΔH values enabled assessment of protein stability across different formulations and batches.
Conformational Insights: DSC provided insights into conformational changes, aiding in the detection of structural alterations due to modifications or degradation.
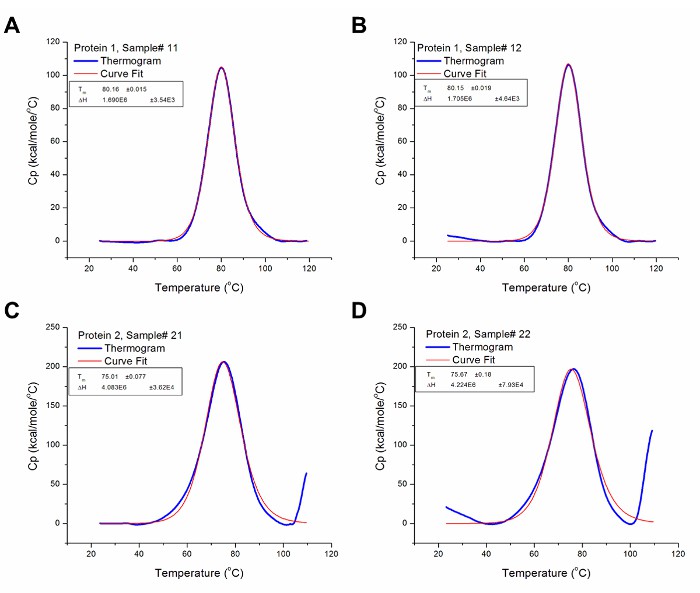 Analyzed DSC Data.
Analyzed DSC Data.Implications
The study highlighted DSC as a powerful tool for:
Quality Control: Ensuring consistency in protein formulations by detecting conformational changes.
Formulation Development: Guiding the selection of stable formulations by comparing thermal stability profiles.
Regulatory Compliance: Providing critical data for regulatory submissions by demonstrating product stability.
This case study underscores the utility of DSC in the biopharmaceutical industry for assessing protein stability, ensuring product quality, and supporting regulatory requirements.
References
- Durowoju IB, Bhandal KS, Hu J, Carpick B, Kirkitadze M. Differential Scanning Calorimetry - A Method for Assessing the Thermal Stability and Conformation of Protein Antigen. J Vis Exp. 2017 Mar 4;(121):55262. DOI: 10.3791/55262
- Colombo A, Ribotta PD, León AE. Differential scanning calorimetry (DSC) studies on the thermal properties of peanut proteins. J Agric Food Chem. 2010 Apr 14;58(7):4434-9. DOI: 10.1021/jf903426f
Related Services
Support Documents
Brochures
KNOWLEDGE CENTER