The disulfide bond plays an important role in maintaining the spatial three-dimensional structure of peptides and proteins and the biological activity determined thereby, and is also related to the stability and renaturation of proteins. Analysis of disulfide bonds and free sulfhydryl groups on proteins helps to analyze the high-level structure of proteins to reveal the biological functions of proteins. Disulfide bond analysis can analyze the structural characteristics of protein to improve its degradation stability and prolong its efficacy. The analysis of disulfide bonds in proteins is an important biochemical technology for biotherapeutic drug development projects. Related technical methods can be used to determine the location and content of disulfide bonds of biopharmaceutical proteins or other biological agents, thereby determining the structure and relative molecular content of proteins, and also measuring free thiol groups.
Creative Proteomics is a biotherapeutic drug partner with rich and professional protein structure research technology. We use first-class experimental equipment and mature experimental technical methods to provide you with reliable one-stop service. The analysis of protein structure characterization information in biopharmaceuticals is an important part of the quality control of peptide and protein biopharmaceuticals. In particular, the ICH Q6B guideline requires the provision of disulfide bond characterization analysis information in protein structures of biopharmaceuticals. We will provide you with comprehensive and GLP / cGMP-compliant N-terminal sequencing analysis service around the ICH guidelines (especially ICH Q6B) and the US FDA issues 'Points to Consider' document.
We Can Provide but Not Limited to:
- Analysis of localization of disulfide bonds in proteins
- Analysis of the proportion of disulfide bonds in proteins
- Analysis of primary structure characteristics of protein
- Analysis of free sulfhydryl groups in proteins
- Protein molecular mass analysis
Technology Platform of Disulfide Bridges & Free Sulfhydryl Groups Analysis Service:
Creative Proteomics provides techniques such as MS In-Source Dissociation, fluorescence analysis, and HPLC combined with derivation techniques to detect the disulfide bonds and free sulfhydryl groups of protein samples.
We provide the following analysis methods:
1) Characterization of protein disulfide linkages by MS in-source dissociation
After digesting the protein sample, the disulfide bonds can be cleaved in different gas phase fragments. ISD can cleave the disulfide bonds within and between the peptide main chains of the protein to generate multiple fragment ions, and then analyze disulfide bonds of proteins by LC-MS/MS. The specific workflow is as follows.
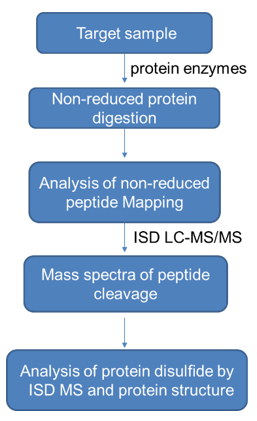
2) Analysis method of free sulfhydryl groups
- Fluorescence analysis method
The free sulfhydryl groups react with iodoacetic acid to form a disulfide bond, and then modified with halogen-containing fluorescent derivatization reagent 5-IAF to generate a strong fluorescent substance, followed by thiol fluorescence detection, showing the change of content of free sulfhydryl groups. The specific workflow is as follows:
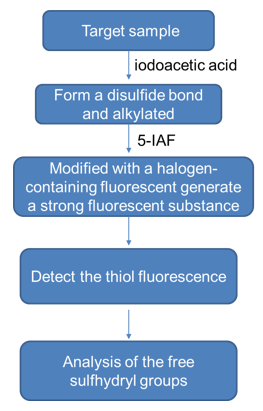
- High performance liquid chromatography analysis method
The free sulfhydryl groups in the sample undergo an alkylation reaction with the new derivatization reagents 12C iodoacetic acid and 13C iodoacetic acid. After separation by liquid chromatography and detection of cysteine by MS , two partially overlapping molecular ion peaks are obtained, and the content of free sulfhydryl groups can be obtained by calculating the peak difference. The specific workflow is as follows:
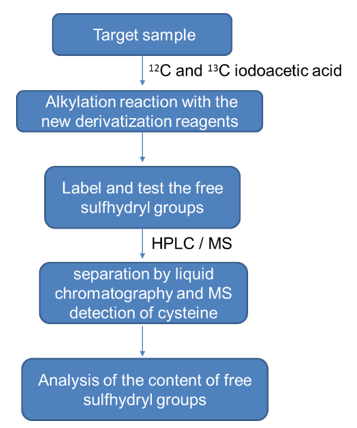
Advantages of Disulfide Bridges & Free Sulfhydryl Groups Analysis Service:
- Short time-consuming: The service uses advanced experimental equipment and uses professional and reliable experimental technology, and the sample preparation and experimental operation steps are simple, which greatly shortens the time of experimental operation and data analysis.
- High sensitivity: Fluorescence analysis has high selectivity and sensitivity, and derivatives have high temperature stability and derivatives have an enhanced effect on the signal, which can reduce the detection limit.
- High-throughput: The combination of HPLC method and derivative technology makes the sample applicable to a wider range.
- Rapid turnaround time: 5-7 days to provide detailed technical reports.
- Customized service: We can customize professional solutions for you according to your research plan needs. You can select or suggest the required items for analysis.
Creative Proteomics' professional researchers can provide customers with a comprehensive analysis of protein disulfide bonds and free sulfhydryl groups to achieve protein structure characterization information and biological function and stability. We will provide you with the complete experimental operation plan, the final disulfide bond analysis information in the protein, the data analysis report of the free thiol group and other data reports. We are committed to providing you with expertise and services in analyzing the structure of biotherapeutic drugs to help you accelerate the pace of research.
References
- Li, X., Yang, X., et al. Characterization of Protein Disulfide Linkages by MS In-Source Dissociation Comparing to CID and ETD Tandem MS. Journal of The American Society for Mass Spectro.metry, 2018.
- Mangold C M T , Werr M , et al. Analysis of intermolecular disulfide bonds and free sulfhydryl groups in hepatitis B surface antigen particles. Archives of virology, 1997.






