- Service
- FAQ
- Demo
- Case Study
- Related Services
- Inquiry
What Is N-Glycan Analysis?
N-glycans are carbohydrate structures covalently attached to proteins at Asn-X-Ser/Thr motifs (where X ≠ Proline), forming N-linked glycosylation. These glycans typically consist of a common core (GlcNAc₂Man₃) and can be extended or branched through Golgi-mediated trimming and addition of monosaccharides. The enzyme PNGase F is widely used to cleave nearly all types of N-glycans—including high-mannose, hybrid, and complex forms—releasing the glycan and converting the Asn residue into Asp.
N-glycans profoundly influence protein folding, stability, immunogenicity, and biological function. For example, in therapeutic antibodies (IgG1), N-glycans in the Fc region modulate effector functions such as ADCC (antibody-dependent cell-mediated cytotoxicity), CDC (complement-dependent cytotoxicity), and pharmacokinetics.
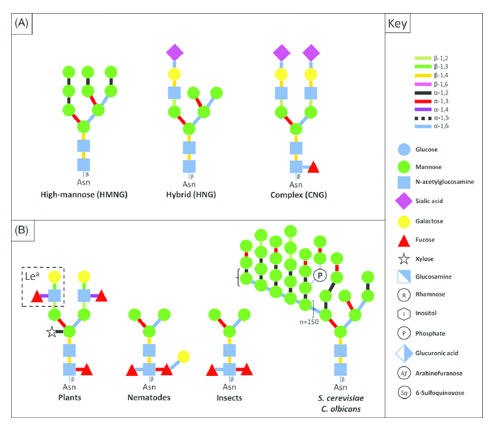 Eukaryotic N-glycan structures (Ladevèze Simon et al. 2016).
Eukaryotic N-glycan structures (Ladevèze Simon et al. 2016).Why N-Glycan Analysis Matters to You
For biopharma and CRO teams, N-glycan profiling is essential because it directly influences therapeutic protein quality and functional performance. Key benefits include:
1. Ensuring product consistency & comparability
Therapeutic monoclonal antibodies (mAbs) exhibit significant batch-to-batch glycan heterogeneity — up to 30 distinct glycoforms on IgG Fc domains, including complex structures like G0F, G1F, and G2F. Rigorous N-glycan profiling is thus vital for production consistency and regulatory-grade comparability .
2. Fine-tuning mAb functionality
Glycan structures influence antibody effector functions. For example, afucosylated antibodies show increased ADCC activity via enhanced FcγRIIIa binding. Variations in terminal galactose or sialic acid residues also modulate CDC, PK half-life, and immune clearance.
3. Supporting clone and process screening
During clone selection and bioprocess development, N -glycan profiling acts as a quality check, where minor changes in culture conditions or cell lines can shift glycosylation patterns — impacting efficacy and immunogenicity.
4. Enabling biosimilar development
For biosimilar candidates, demonstrating glycan profile equivalence to reference biologics is a regulatory prerequisite.
N-Glycan vs. O-Glycan: Defining the Right Service
Understanding the distinction between N-linked and O-linked glycosylation is essential for choosing the right analytical approach:
Structural & Site Differences
N-glycans are covalently attached to asparagine in the Asn–X–Ser/Thr motif (X ≠ Proline) via a pre-assembled oligosaccharide transferred en bloc during protein synthesis in the ER.
O-Glycans, by contrast, are added one sugar at a time to the hydroxyl group of serine or threonine residues—without a defined consensus motif—and their initiation occurs typically in the Golgi.
Size & Complexity
N-glycans often include 8–20 monosaccharides, can branch extensively (tri- or tetra-antennary), and may carry sialic acids and fucose.
O-Glycans are generally shorter (3–6 residues), less branched, and exhibit higher structural diversity in their core structures.
Analytical Implications
Release methods differ: N -glycans are typically released enzymatically (e.g., PNGase F), while O-Glycans often require chemical release (e.g., beta-elimination).
Analytical platforms and bioinformatics pipelines must be optimized for either type due to differences in chemical structure and structural diversity.
In short, if your protein contains Asn–X–Ser/Thr motifs and you need detailed branching, site, and fucosylation/sialylation profiling, choose our N -glycan analysis service. For more diverse O-linked glycosylation patterns, consider our O-Glycans profiling service.
N-Glycan Profiling Services Backed by Regulatory-Ready Expertise
At Creative Proteomics, we offer more than just analytical support—we deliver end-to-end glycan characterization tailored for the pharmaceutical industry. Backed by robust biopharmaceutical platforms and a deep understanding of regulatory expectations, our team ensures every N-glycan analysis meets both technical and compliance standards.
Our workflows are aligned with the latest ICH Q6B guidelines—ensuring your data supports global regulatory filings.
What We Deliver:
Our N-glycan analysis services cover a full spectrum of research and quality assurance needs:
- Quantitative profiling of glycan isomers for product consistency
- Detailed mapping of monosaccharide linkages
- Structural elucidation of N-linked oligosaccharide chains
- Quality assessments for batch-to-batch glycan variation
- Individual glycoform distribution and abundance analysis
- Sequencing and composition of oligosaccharide chains
Whether you're developing a monoclonal antibody or screening biosimilar comparability, we provide results that integrate seamlessly into your CMC documentation and regulatory dossiers.
Advanced Technology Platforms for N-glycan Analysis
Core Analytical Technologies
We employ a diverse suite of orthogonal methods to deliver both structural detail and quantitative insight:
N-glycan release technology: Enzymatic cleavage using PNGase F ensures efficient and specific release from glycoproteins
Fluorescent labeling: Tags such as 2-aminobenzamide (2-AB) enhance detection sensitivity in downstream analyses
Hydrophilic Interaction Liquid Chromatography (HILIC): Separates labeled glycans based on polarity for reproducible GU assignment
Reversed-phase HPLC (RP-HPLC): Used for peptide separation and glycopeptide enrichment
Anion exchange HPLC (HPAEC-PAD): Offers precise monosaccharide and linkage resolution
Mass spectrometry (MS): Provides glycan composition, branching, and isomer detection at the molecular level
Two Proven Analytical Workflows
1. Fluorescence-Based N-glycan Profiling
Step 1: Release N-glycans from glycoproteins using PNGase F
Step 2: Enrich released glycans via solid-phase extraction
Step 3: Label glycans with 2-AB or similar fluorescent dye
Step 4: Separate using HILIC chromatography
Step 5: Detect using fluorescence detection to produce high-resolution N-glycan fingerprints

Ideal for: monoclonal antibody batch comparison, biosimilar characterization, and glycoform consistency.
2. Glycopeptide Mass Spectrometry Workflow
Step 1: Digest proteins using proteases to generate glycopeptides
Step 2: Enrich glycopeptides via RP-HPLC
Step 3: Separate glycans using anion exchange chromatography
Step 4: Analyze with combined HPAEC-PAD and MS to resolve glycan structure, composition, and site specificity
 The workflow of the Glycopeptide Mass Spectrometry technology
The workflow of the Glycopeptide Mass Spectrometry technologyIdeal for: glycosylation site mapping, structural isomer differentiation, and complex glycoprotein profiling.
Why Choose Us
Multi-Platform Technical Expertise
- MALDI-TOF MS: Enables rapid profiling of N-glycans, including permethylated and sialylated forms, with high sensitivity and batch-level consistency.
- HILIC-UHPLC-MS/FLD: Delivers detailed separation and quantitation via GU-based profiling—ideal for comparing multiple batches or biosimilar candidates.
- CE-MS: Specializes in resolving low-abundance and isomeric glycans, using minimal sample input
Expert Scientific Support
- Our team of analytical chemists and mass spectrometry specialists brings decades of experience across biologics and glycomics projects, offering troubleshooting and workflow optimization.
- We collaborate closely with research teams to align assay design with specific study goals, ensuring reliable and actionable results.
Flexible & Customized Services
- Tailored service configurations based on sample type, throughput needs, desired structural depth, and platform preference.
- We accommodate diverse sample types—from mAbs and biosimilars to plasma or cell lysates—adapting to your research requirements and timelines.
Sample Requirements
Our N-glycan Analysis Service supports a wide range of sample types and research applications. Below are examples tailored for both pharmaceutical development and academic research:
Sample Sources
| Sample Type | Applications |
|---|---|
| Therapeutic mAbs / Fusion Proteins |
|
| Biological Fluids |
|
| CHO Cell Culture Supernatants |
|
| Recombinant Glycoprotein Samples |
|
| Sample Type | Minimum Amount | Preparation Guidelines |
|---|---|---|
| Purified proteins/antibodies | 10–30 µg | Use non-denaturing buffer (e.g., PBS, Tris), avoid glycerol, salts, detergents |
| Plasma / Serum / Cell lysates | ≥5–10 µL or µg protein | Clarify before freezing; store at –80 °C; use protease inhibitors where applicable |
| CHO culture supernatant | ≥100 µL (clarified) | Centrifuge to remove debris; submit frozen |
| Fusion proteins / Recombinants | 10–30 µg | Submit in buffer without interfering agents |
| FFPE / Formalin-fixed proteins | Based on extract yield | Contact us for consultation on compatibility and preprocessing |
✅ Submission Tips
- Avoid freeze–thaw cycles
- Use low-retention tubes, properly labeled
- Include documentation: sample ID, concentration, and intended analysis
One-Stop Analytical Delivery
Every project includes:
- A complete sample-to-report workflow
- Raw and processed chromatograms
- Quantified glycan tables (relative and absolute abundances)
- Structural annotations for glycoform identity and heterogeneity
Application Scenarios
1. Therapeutic Antibody Development
Detailed glycan profiling informs key quality attributes.
Case Study Insight: A comparative analysis of four mAbs using ten workflows revealed extensive branching and fucosylation heterogeneity, reinforcing the need for comprehensive profiling during process development.
Functional Relevance: Fc N-glycans modulate effector functions—such as ADCC, CDC, and ADCP—while core-fucose removal enhances ADCC activity via stronger FcγRIIIa binding.
2. Clone Selection & Bioprocess Optimization
Glycan patterns are sensitive to culture media and metabolic flux.
Example: Metabolic flux analysis predicting UDP-Gal levels correlates with galactosylation profiles (e.g., G0F, G1F, G2F), offering guidance on media formulation.
3. Biosimilar Comparability
High-resolution profiling ensures structural equivalence and regulatory alignment.
Glycan similarity is critical for regulatory acceptance—even minor structural differences can impact biosimilarity.
4. Academic & Biomarker Discovery
Analyzing glycoproteins or biofluids for research-led insights.
Glycan changes in plasma or tissue lysates can serve as research markers or support fundamental biological studies.
Why Our Service Works for These Scenarios
- Multi-platform connectivity (MALDI, HILIC, CE-MS) ensures coverage of diverse glycan types.
- High sensitivity enables detection of low-abundance structures and heterogeneity.
- Quantitative and structural depth
Frequently Asked Questions
Q: What sample types do you accept?
A: We support a wide variety of samples, including:
- Purified proteins or antibodies
- Plasma, serum, cell lysates
- CHO cell culture supernatants
- Recombinant and fusion proteins, including FFPE-derived extracts
This aligns with industry standards for N-glycan profiling.
Q: What is the minimum sample amount required?
A: Typically:
Purified protein/antibody: ≥10–30 μg
Biological fluids (plasma, lysate): ≥5–10 μL or μg
This is consistent with workflows used in released glycan studies requiring as little as 15 μg protein
Q: Which labeling and detection methods do you use?
A: We use enzymatic release with split-flow labeling:
Labels: 2-AB, InstantPC, APTS, RapiFluor-MS
Detection platforms: HILIC-UHPLC-FLD/MS, CE-MS, and/or MALDI-TOF. This combined approach ensures high sensitivity and robust structural resolution.
Q: How are glycans identified and quantified?
A: Released glycans are quantified via fluorescent detection and separated by HILIC columns (often using a dextran ladder for GU assignment). Confirmation is achieved using MS and, when needed, exoglycosidase digestion.
Q: Can low-abundance glycoforms or isomers be detected?
A: Yes. Techniques like CE-MS and MALDI-TOF, along with sensitive labels, allow detection of low-abundance structures such as G1F' isomers and nuanced sialylation patterns.
Q: What deliverables do customers receive?
A: Comprehensive output includes:
- Raw chromatograms and chromatographic peak assignments
- Mass spectra and glycan structural annotations
- Quantitative tables (relative and absolute abundance)
- Summary insights on glycoform heterogeneity and functional implications
Demo Results

Case Studies
✅ Case Study 1: Afucosylated mAbs Enhance ADCC Activity
- Afucosylated IgG1 antibodies—lacking core fucose on their Fc N-glycans—show 10–100× stronger binding to FcγRIIIa than fucosylated counterparts, significantly boosting antibody-dependent cell-mediated cytotoxicity (ADCC) ( https://doi.org/10.3389/fimmu.2022.929895). Ferrara et al. (2011) report that antibodies without fucose achieved markedly higher ADCC in vitro—a critical quality attribute for therapeutic antibodies (DOI: 10.1073/pnas.1108455108).
Implication: Accurate quantification of afucosylated glycoforms is essential for optimizing antibody function and ensuring batch consistency.
✅ Case Study 2: Detecting Trace Isomeric Glycans
- Messina et al. (2020) used HILIC-UPLC-MS with 2-AB labeling to uncover low-abundance glycan isomers—including G1Fa/G1Fb—making up <1% of total glycans in clinical samples(DOI: 10.1007/s10719-020-09947-7).
Using both fluorescence and MS detection in tandem confirms our ability to resolve subtle structural differences.
Implication: Being able to detect trace isomers enhances both product integrity and regulatory reliability.
✅ Case Study 3: Profiling Non-Human Glycans in mAbs
Structural analytics revealed non-human glycans such as α-Gal and Neu5Gc in therapeutic antibodies—a known source of immunogenicity (DOI:10.1016/B978-0-12-821447-3.00018-4).
Early identification of these glycoforms is critical to mitigating safety risks and enhancing product quality.
✅ Case Study 4: Resolving Isomers via Ion Mobility & Spectroscopy
Advanced methods like ion mobility spectrometry combined with cryogenic IR spectroscopy can distinguish positional isomers (e.g., α1–6 vs α1–3 galactosylation) with outstanding sensitivity (~0.2–0.3% isomer ratio difference) (PMID: 28370073).
 Figure 2. (a) RPLC separation of permethylated isomeric O-glycan trisaccharides. (b) MS/MS spectra of isomers in peaks A and B (upper panel).
Figure 2. (a) RPLC separation of permethylated isomeric O-glycan trisaccharides. (b) MS/MS spectra of isomers in peaks A and B (upper panel).Implication: This level of resolution aids in precise biosimilar comparability and nuanced structure-function insights.
Why These Matter for N-glycan Services:
- Quantitative profiling of functional glycoforms (e.g., afucosylated species)
- Detection of trace or isomeric glycans for regulatory compliance
- Identification of immunogenic, non-human structures to support safety profiling
References
- Ladevèze Simon, Laville Elisabeth, et al. Mannoside recognition and degradation by bacteria. Biological reviews of the Cambridge Philosophical Society. 2016. DOI: 10.1111/brv.12316
- Higel F, Demelbauer U, et al. Reversed-phase liquid-chromatographic mass spectrometricN-glycan analysis of biopharmaceuticals. Analytical & Bioanalytical Chemistry, 2013, 405(8): 2481-2493. DOI: 10.1007/s00216-012-6690-3
Related Services