Sumoylation is a classic protein post-translational modification biotechnology in protein drug development and production. Sumoylation Analysis can be used for enriching modified peptides, analyzing peptide mass spectra, identifying modification sites of proteins (peptides), quantitative analysis of SUMO-proteins (peptides), and identifying SUMO-modified cells and organs difference.
SUMO modification (Small Ubiquitin-like Modifiers) is a kind of post-translational modification of protein. It has a variety of modification and processing forms, and there are many types. SUMO modification mainly plays an important role in the assembly of nuclear proteins, maintaining genome integrity, and regulating intracellular signaling pathways. Mature protein drugs undergo a series of post-translational modifications and have specific biological activities. Naturally modified protein drugs need to determine the type of modification, identify the site of modification, and analyze the structure of the modification. And its specificity, accuracy and stability were measured. Therefore, according to the SUMO ubiquitination modification of the protein or polypeptide under test, the biological characteristics of the protein drug or polypeptide chain can be obtained, so as to reveal the SUMO ubiquitination modification sites and structures of the protein or polypeptide.
According to ICH Q6B guideline, Creative Proteomics will perform a high-quality Sumoylation analysis services to identify modification sites of proteins (peptides), quantitatively analyze SUMO-proteins (peptides), and have better understanding of the analytical characterization of structure, comparability, stability, pharmacogenetics, and so forth. The Sumoylation analysis service can be provided according to PharmEu and USP<1047>.
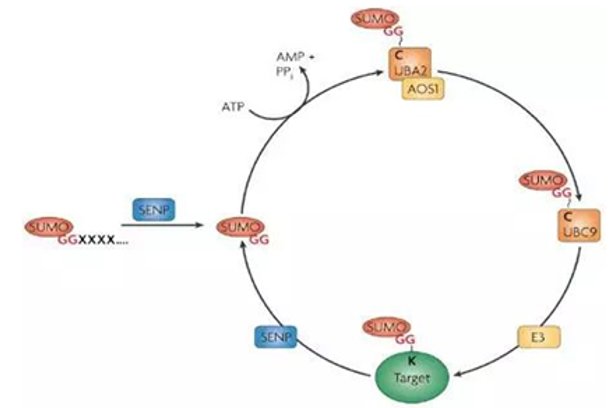
Sumoylation decorates the schematic (Hendriks, et al. 2018).
We Can Provide but Not Limited to:
- Identify the SUMO content of proteins / peptides
- Identification of SUMO modification sites of proteins / peptides
- Analysis of SUMO-modified structures of proteins / peptides
- Qualitative analysis of SUMO proteins / peptides
- Stability analysis of proteins modified with SUMO
The Workflow of Sumoylation Analysis
Advantages of Sumoylation Analysis Service
- High-performance mass spectrometry system: equipped with AXIMA series shimazu new MALDI 7090TM, LCMS-IT-TOF high-end mass spectrometers. Using the combination of ion trap mass spectrometry and TOF mass spectrometry, multistage mass spectrometry can be conducted at the same time, effectively improving the quantitative and qualitative analysis of post-translational modification of protein drugs.
- High resolution, high-quality accuracy, and high sensitivity.
- High flux and fast polarity conversion: positive and negative polarity conversion with only 100 m/s.
- The unique ion trap import and export technology can reduce the ion loss or low and maintain the high-quality spectrum in grade 10 mass spectrometry.
- Rapid turnaround time: 5-7 days to provide comprehensive report.
Creative proteomics analytical scientists can provide a clear, concise written report to help customers detect the SUMO protein drug (peptide) modification sites, analyze the SUMO modification structure, etc.
References
- Hendriks Ivo A, et al. Site-specific characterization of endogenous SUMOylation across species and organs. Nat Commun. 2018.
- Yan liu, et al. An improved method for identifying SUMOylation sites of viral proteins. Virologica Sinica. 2017.






