Enzymes are biological macromolecules with biocatalytic activity in living organisms, including proteins and nucleic acids. Most enzymes are composed of proteins or complexes of proteins and coenzymes by nature. Due to the high specificity and catalytic efficiency, enzymes are highly targeted and effective in disease treatments of all kinds. Currently, more than 2,000 enzymes have been discovered in the biological world, and among these, there are about 50-60 industrial enzymes; over 120 therapeutic and diagnostic enzymes; over 300 reagent enzymes; and over 100 enzymes were used as genetic engineering tools.
Classification and application of medicinal enzymes:
- Digestive enzymes
- Anti-inflammatory enzymes
- Enzymes related to the treatment of cardiovascular and cerebrovascular diseases
- Anti-tumor enzymes
- Enzymes related to biological redox electron transfer
- Other medicinal enzymes
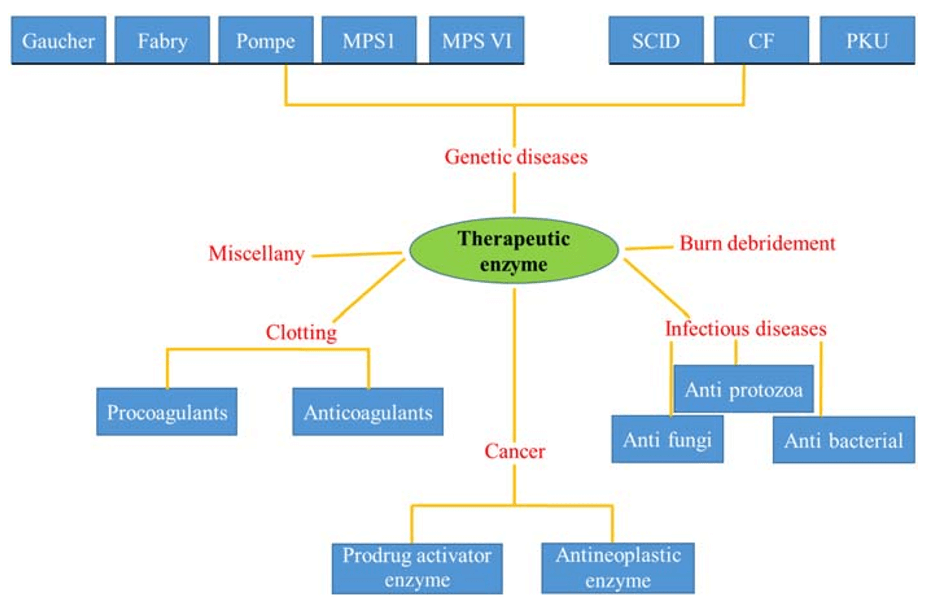 Application of therapeutic enzymes in different disorders and diseases [Carlos Simões Nunes Ph.D., 2018]
Application of therapeutic enzymes in different disorders and diseases [Carlos Simões Nunes Ph.D., 2018]Why Characterize Therapeutic Enzymes?
Regulatory agencies strictly govern the testing and production of therapeutic enzymes worldwide, and batch-to-batch consistency is crucially important. Batch manufacturing begins from the fermentation or cell culturing stage. Subsequently, therapeutic enzymes are expressed in a cell culture bioreactor to robust the reproducible protein purification or downstream processing (DSP) of the target product. Thus, it is critical to ensure the safe delivery of these medicines throughout the whole process. QC is highly crucial in therapeutic enzyme production to ensure the drug's identity, strength, potency, purity, and safety. As a result, QC that are capable of operating across the entire biopharmaceutical process workflow, evaluating novel enzymes as well as upgrading and improving existing ones are current needs.
Our Therapeutic Enzymes Characterization Services
Creative Proteomics' comprehensive suite of protein analysis services brings together a variety of methods, including amino acid analysis, concentration/yield assays, cell potential and in vitro cell culture, glycosylation assays, post-translational modifications, etc.
Peptide identification analysis (LC-MS/MS)
LC-MS/MS analysis is well-suited for the identification and detailed characterization of single or multiple peptides in a complex solution.
High-resolution molecular weight analysis (electrospray mass spectrometry)
Peptide sequencing
- N-terminal sequence analysis
- C-terminal sequence analysis
- Sequence coverage
- HPLC peptide mapping
Purity and impurities analysis
- Residual organic solvent determination per USP <467>
- Purity/Potency assessment by HPLC
- Host cell protein analysis
- Purity assessment by capillary electrophoresis (CE, CGE, cIEF)
Others
- Clinical samples preparation/testing for pharmacodynamics and pharmacokinetics studies using multiple reactions monitoring and LC-MS/MS
- Forced degradation studies
Advantages
- Capable of identifying and characterizing target molecules of >0.01%, low-level impurities
- Unique sequence analysis tools bring additional values
- 100% quality control
Creative Proteomics strictly complies with the FDA and ICH guidelines to provide you with the most comprehensive antibody-related analysis services. In Creative Proteomics, a one-stop solution is tailored for your programs. We are confident in providing the most helpful technical support for drug discovery, manufacturing processes, and other special requirements. Please feel free to contact us if you have any further questions, and we look forward to cooperating with you.
Reference
- Nikolaos Labrou. Therapeutic Enzymes: Function and Clinical Implications. 2019.






