- Backgrourd
- Services
- FAQ
- Demo
- Case Study
- Related Services
- Support Documents
- Inquiry
In today's multi-billion dollar antibody market, having a working hybridoma cell line isn't enough. Whether you're facing risks like freezer failure, contamination, or genetic drift, one thing is clear—your antibodies need a backup plan.
Antibody sequencing offers that safety net. By determining the precise genetic sequence of your antibody, you gain full control over its reproducible, recombinant production—on-demand, whenever and wherever it's needed.
But the value of sequencing goes far beyond emergency preparedness.
Why Sequence Your Antibody?
Secure Your IP: Accurate sequence data is a prerequisite for patent applications and intellectual property protection.
Enable Recombinant Expression: With the gene sequence in hand, recombinant antibodies can be reliably produced—eliminating reliance on fragile hybridoma cultures.
Support Engineering Workflows: Sequencing unlocks downstream applications such as:
- Isotype or subtype switching
- Fc region optimization
- Antibody humanization for clinical development
Mass Spectrometry-Based Antibody Sequencing
Lost hybridoma cell lines don't have to mean lost antibodies. Thanks to advancements in high-resolution mass spectrometry, full-length monoclonal antibody (mAb) sequencing is now possible—even without the original cells.
If you still have purified antibodies in hand, our mass spectrometry-based platform can recover their complete sequence. This is especially critical in drug development and diagnostics, where monoclonal antibodies drive innovation across therapeutic and detection applications.
At the core of each antibody's function lies its primary structure, particularly the complementarity-determining regions (CDRs). These amino acid sequences govern antigen recognition and binding strength. That's why fast, precise sequence analysis of the entire antibody—especially the variable region—is key to preserving its function, engineering improvements, and enabling recombinant expression.
Our Antibody Sequencing Platform: Speed Meets Accuracy
Creative Proteomics leverages the Orbitrap Fusion Lumos, one of the world's highest-resolution mass spectrometry instruments, to deliver next-generation antibody sequencing with unmatched accuracy and depth.
What We Offer:
Recover the complete amino acid sequence of both heavy and light chains—essential for recombinant expression and biosimilar design.
- Variable Region (CDR) Analysis
Pinpoint the highly variable complementarity-determining regions (CDRs) that define antibody specificity and function.
Reconstruct full monoclonal antibody sequences from protein samples—without needing prior genetic data. Ideal for lost hybridoma lines or poorly documented antibodies.
Detect and profile C-terminal lysine heterogeneity—a common quality attribute in therapeutic antibodies that can impact efficacy and stability.
- Subtype & Species Flexibility
Compatible with various antibody isotypes (IgG, IgM) and formats, including fluorescent-labeled, immobilized, or cross-species antibodies.
- In-House Lab for IP Security
All sequencing is conducted independently at our facility, ensuring strict confidentiality and full control over your intellectual property.
- Expert Bioinformatics Support
Our advanced sequence assembly algorithms and curated databases enable rapid, high-confidence results for even complex antibody formats.
Advantages of Our Antibody Sequencing Service
At Creative Proteomics, we've built our antibody sequencing platform to deliver the depth, accuracy, and flexibility today's biopharma projects demand.
Complete Sequence Coverage
Our optimised high-resolution MS workflow, paired with industry-leading de novo sequencing algorithms, ensures full-length sequence recovery—from the N-terminus to the C-terminus.
Unmatched Accuracy
Each amino acid is confirmed using redundant MS/MS spectral data from overlapping peptides, delivering base-by-base confidence in sequence integrity.
Broad Format & Species Compatibility
We can accurately sequence:
- All major antibody types: IgG, IgA, IgM, IgY, Fab, ScFv
- Multiple species: human, mouse, rat, rabbit, hamster
- Challenging samples: conjugated antibodies, mixtures, and antibodies with dual light chains
High-Throughput Capabilities
Whether you're analyzing a single clone or running a 100-antibody screen, our scalable workflows adapt to meet your volume—without compromising quality.
Sample Requirements
To begin your project, we require:
≥50–100 µg of purified monoclonal antibody
Service Workflow: How Our Sequencing Process Works
Sample QC & Preparation
We assess purity and concentration. Optional pre-treatment if the antibody is conjugated or mixed.
Enzymatic Digestion
Proteins are digested into overlapping peptides for optimal MS coverage.
High-Resolution LC-MS/MS
Peptides are analyzed using Orbitrap Fusion Lumos for in-depth fragmentation data.
Sequence Assembly & Variant Detection
Advanced algorithms reconstruct full-length sequences and identify C-terminal or light chain variants.
Data Review & Reporting
Deliverables include annotated sequences, quality metrics, and expert interpretation.
Deliverables: What You Receive
- Full amino acid sequences (light and heavy chains)
- Annotated CDR regions and post-translational modifications
- Sequence confidence metrics (coverage % and MS/MS support)
- Raw MS data (optional)
- Publication-ready report (PDF)
- Sequence FASTA files for cloning or expression
Turnaround Time & Project Capacity
Project Turnaround
Our antibody sequencing service is delivered in accordance with ICH Q6B specifications for characterisation of biotechnological/biological products, ensuring analytical consistency and regulatory alignment.
Project timelines vary based on sample complexity, customization needs, and batch volume. We will provide a detailed schedule and milestone plan upon project initiation.
Flexible Capacity
Whether you're working on a single clone or scaling up for large-volume screening, our platform adapts to your project scope.
We support:
- Early discovery and pilot-stage investigations
- Sequence confirmation for preclinical and IND-enabling studies
- Batch release or QC sequencing for biologics programs
Application Scenarios: When to Use This Service
- Lost or unstable hybridoma lines
- Antibody patent filing and IP disputes
- Antibody humanization or Fc engineering
- Biosimilar sequence verification
- QC for commercial antibody reagents
- Confirming the integrity of conjugated or labelled antibodies
Frequently Asked Questions (FAQs)
Q: Can you sequence antibodies from serum or impure samples?
A: For best results, we recommend purified antibodies. However, we can evaluate impure or partially degraded samples on a case-by-case basis.
Q: Is sequence data kept confidential?
A: Absolutely. All sequencing is performed in-house under strict confidentiality agreements. Your data is never shared or stored beyond your project scope.
Q: Can I use the sequence data for recombinant expression or patent filing?
A: Yes. Our sequences are validated for downstream expression and suitable for regulatory and IP submissions.
Q: Do you provide assistance with antibody expression after sequencing?
A: While our core service is sequencing, we partner with trusted CROs for downstream expression and optimization upon request.
Demo
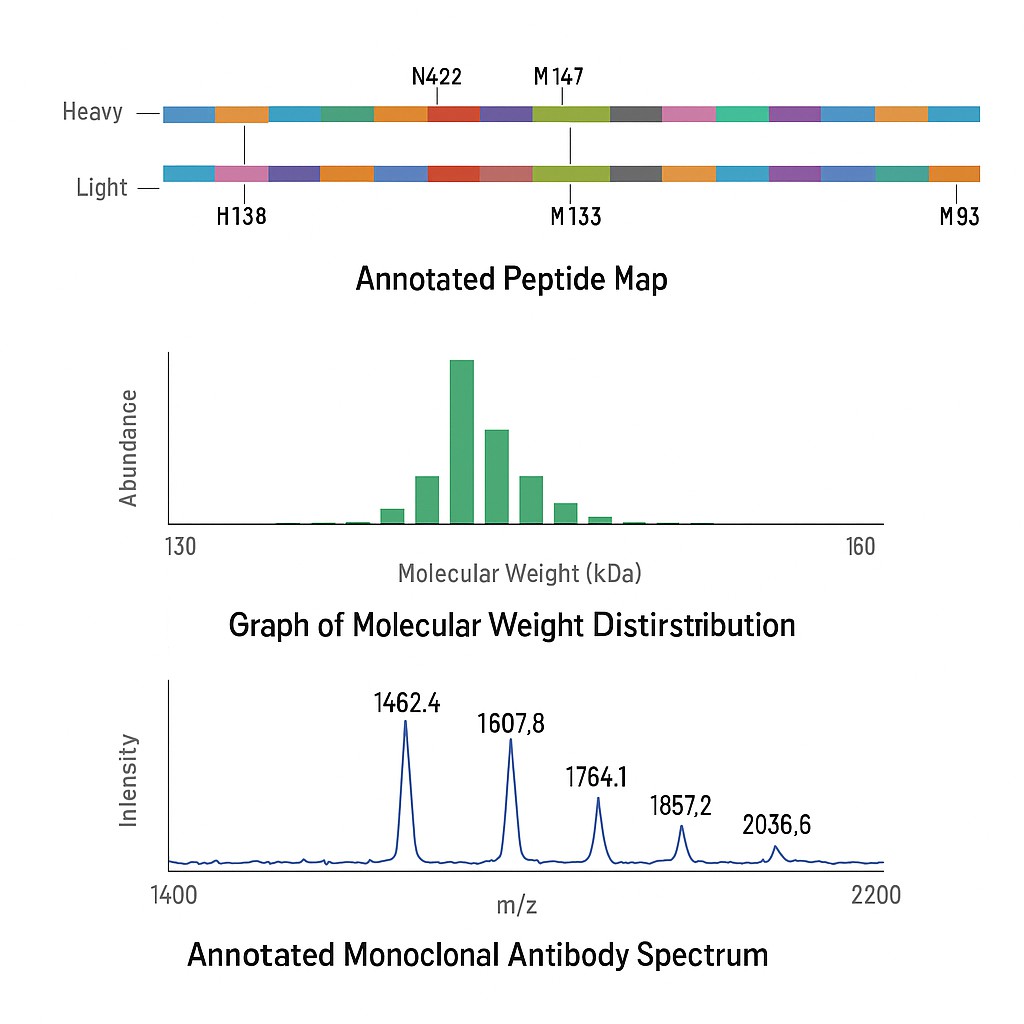
Case Study: Comprehensive Sequence Characterization of a Monoclonal Antibody Using a Nonspecific Immobilized Enzyme Reactor
Project Objective
To achieve comprehensive and unambiguous amino acid sequence characterization of a therapeutic monoclonal antibody (mAb) using a single analytical run, leveraging a nonspecific immobilized enzyme reactor coupled with advanced mass spectrometry techniques.
Experimental Overview
Sample Preparation:
- The mAb was reduced to separate heavy and light chains.
- Digestion was performed using an immobilized Aspergillopepsin I enzyme reactor, producing a broad range of peptide fragments.
Mass Spectrometry Analysis:
- Utilized a Thermo Scientific Orbitrap Fusion mass spectrometer.
- Employed both Electron Transfer Dissociation (ETD) and Higher-energy Collisional Dissociation (HCD) fragmentation methods.
- Data-dependent acquisition allowed for optimal fragmentation based on peptide size and charge.
Data Processing:
- Advanced bioinformatics tools were used to assemble the peptide sequences and map them to the full-length antibody sequence.
- Composite fragment ion coverage maps were generated to visualize sequence coverage.
Results Summary
- Achieved 100% sequence coverage for both heavy and light chains of the mAb in a single chromatographic analysis.
- Successfully identified and localized post-translational modifications (PTMs), including glycosylation sites.
- Demonstrated the capability to distinguish between isobaric amino acids, such as leucine and isoleucine, enhancing sequence accuracy.
 It illustrates the composite fragment ion coverage maps from pooling identified cleavages for the light chain, heavy chain Fd', and heavy chain Fc/2 of the monoclonal antibody.
It illustrates the composite fragment ion coverage maps from pooling identified cleavages for the light chain, heavy chain Fd', and heavy chain Fc/2 of the monoclonal antibody.Value for Biologics Development
This study exemplifies the power of integrating a nonspecific immobilized enzyme reactor with advanced mass spectrometry to obtain a complete and accurate sequence of monoclonal antibodies. The approach is invaluable for therapeutic antibody development, ensuring structural integrity and aiding in regulatory submissions.
Reference
- Hinkle, J. D., D'Ippolito, R. A., Panepinto, M. C., Wang, W.-H., Bai, D. L., Shabanowitz, J., & Hunt, D. F. (2019). Unambiguous Sequence Characterization of a Monoclonal Antibody in a Single Analysis Using a Nonspecific Immobilized Enzyme Reactor. Analytical Chemistry, 91(21), 13547–13554. doi: 10.1021/acs.analchem.9b02666
Related Services
Support Documents