S-prenylation controls many proteins membrane targeting many proteins, which is a post-translational lipid modification, of which usually one walk in an integrated mechanism to specific membranes that underlying fundamental cellular signaling pathways and diseases like cancer and microbial infections. The small G proteins (small GTPases), encompasses Ras, are the largest group of S-prenylated proteins, Rho and Rab families of proteins. Other proteins like heterotrimeric G protein γ subunits and nuclear lamins also need S-prenylation for proper signaling functions. Misregulation of protein S-prenylation on many substrates often causes major defects in cell signaling and disease progression. The precursors for S-prenylation are originated from isoprenoid pyrophosphate substrates. Mammalian Gα subunits are either myristoylated or palmitoylated or modified by both lipids. The other signal is offered either by basic residues near the C-terminus in a hypervariable region, or by palmitoylation, a modification that requires previous prenylation, of this region. Prenylation is part of a consecutive intracellular trafficking mechanism for the heterotrimeric G proteins. Prenylation of Gγ subunits of Gβγ dimers targets them to the ER where they form heterotrimers with Gα subunits. For heterotrimeric G proteins, the dual signals for transit to the plasma membrane appears to be intimately tied to heterotrimer formation where prenylation is on the Gβγ dimer, and the other signal, myristoylation or palmitoylation, is on the Gα subunit.
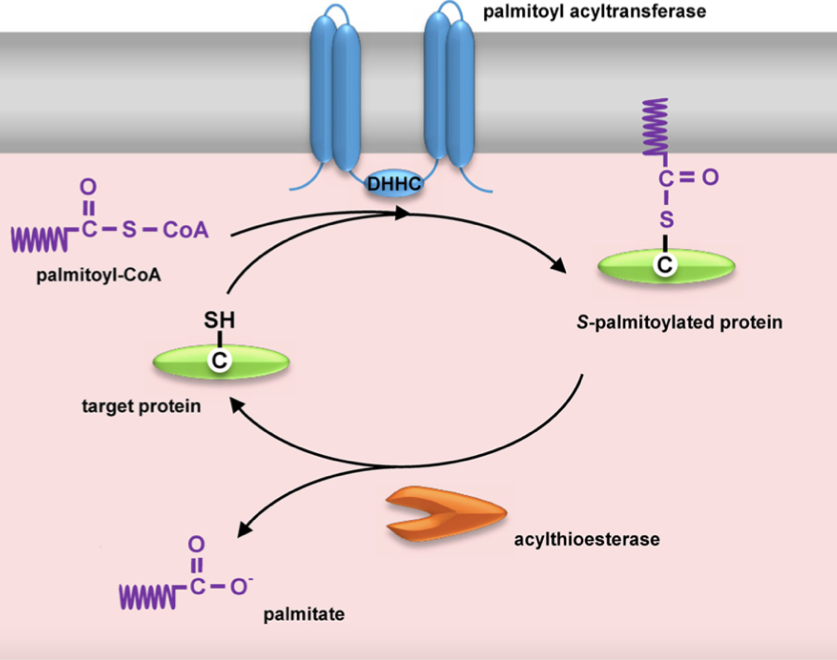 Figure 1: Dynamic protein S-palmitoylation.
Figure 1: Dynamic protein S-palmitoylation.For protein prenylation, the analysis needs 25–100 µg of purified recombinant protein, which can be conducted with 1–25 µg or more of purified recombinant protein. What should be noticed is the acyl groups can be easily determined also when using 1 µg of purified protein, and the minimum amount for protein prenylation analysis, 25 µg is needed. The number of starting materials required for the analysis rely on the protein expression levels and thus is submitted to changes between different proteins. Relative quantification of the protein lipidation levels can be achieved provided that protein concentrations and volumes of extraction solutions are equal between different samples.
Creative Proteomics has a strict workflow to analyze S-prenylation to meet your requirements.






