In the absence of antigens, the human immune system is capable of producing over 10^12 distinct antibodies. This capacity increases to as high as 10^18 kinds when foreign antigens are present. The mammalian immune system has concurrently evolved numerous mechanisms such as gene segment joining and somatic hypermutation to generate a repertoire of antibodies, exceeding the number of their genes (Table 1). These mechanisms contribute to the diversity of antibodies, enabling the immune system to swiftly detect and neutralize pathogens and regulate immune responses accordingly.
Table 1. Number of functional human immunoglobulin gene segments in the heavy and light chain locus.
| Immunoglobulin (Ig) gene segments | |||||||
| Gene locus | Ig chain | Chromosomal location | Locus size (kb) | Variable (V) | Diversity (D) | Joining (J) | Constant (C) |
| IGH | Heavy chain | 14q32.33 | 1250 | 38–46 | 23 | 6 | 9 |
| IGK | κ Light chain | 2p11.2 | 18201 | 34–38 | 0 | 5 | 1 |
| IGL | λ Light chain | 22q11.2 | 1050 | 29–33 | 0 | 4–5 | 4–5 |
What Generates Antibody Diversity?
Before interaction with antigens, B cells undergo development in the bone marrow where a combination of gene segment joining, junctional diversity, and light-heavy chain pairing induce antibody diversity (Figure 1). After antigen stimulation, somatic hypermutation and class-switch recombination also promote antibody diversity within activated B cells. B cells that demonstrate a higher affinity for the target antigen are preferentially selected, thereby promoting affinity maturation.
The complexity of antibody diversification mechanisms necessitates sophisticated means to characterize antibody repertoires at a genomic level. Creative Proteomics is equipped to undertake this task via monoclonal and polyclonal antibody sequencing services, utilizing proteomic methodologies for targeted antibody sequencing. By utilizing our cutting-edge de novo protein sequencing software, we can define antibody libraries and monitor the real-time status of immune responses. Boasting globally leading expertise in protein/antibody de novo sequencing, we are committed to the application of this technology in the development of novel antibodies and drugs, considerably diminishing the timeline of new antibody research and development.
Antibody Locus and VDJ rearrangements
The antibody locus encompasses several gene segments that undergo recombination within developing B cells, thereby constructing a highly variable library of antibodies. The variable region of the light chains (LCs) is constituted by two gene segments, comprising a large variable (V) segment along with a shorter joining (J) segment. The variable region of heavy chains (HCs), on the other hand, is an amalgamation made up of a V segment, a J segment, and a diversity (D) segment.
Figure 1 gives an overview of the respective steps of the V(D)J recombination for construction of the V region of the heavy chain immunoglobulin.
The process of site-specific recombination induces V(D)J recombination, which is initiated by the RAG recombinase enzyme, causing chromosomal DNA double-strand breaks. This enzymatic complex is capable of identifying and binding conservative sequences on the lateral sides of each V, D, and J segments, performing a cut in the DNA at these points. Subsequently, the selected V, D, and J segments are reorganized and linked together, resulting in the formation of V(D)J exons. DNA termini are repaired by DNA repair enzymes, a process that may lead to the deletion or inversion of gene segments.
During genetic recombination, nucleotides are prone to loss or insertion at the junctional sites, leading to frameshift mutations. These mutations induce junctional diversity, thereby generating additional antibody diversity within the third complementarity determining region (CDR3). Following transcription and translation, the formed V(D)J exon can produce functional heavy chains (HC) or light chains (LC) as depicted in Figure 3.
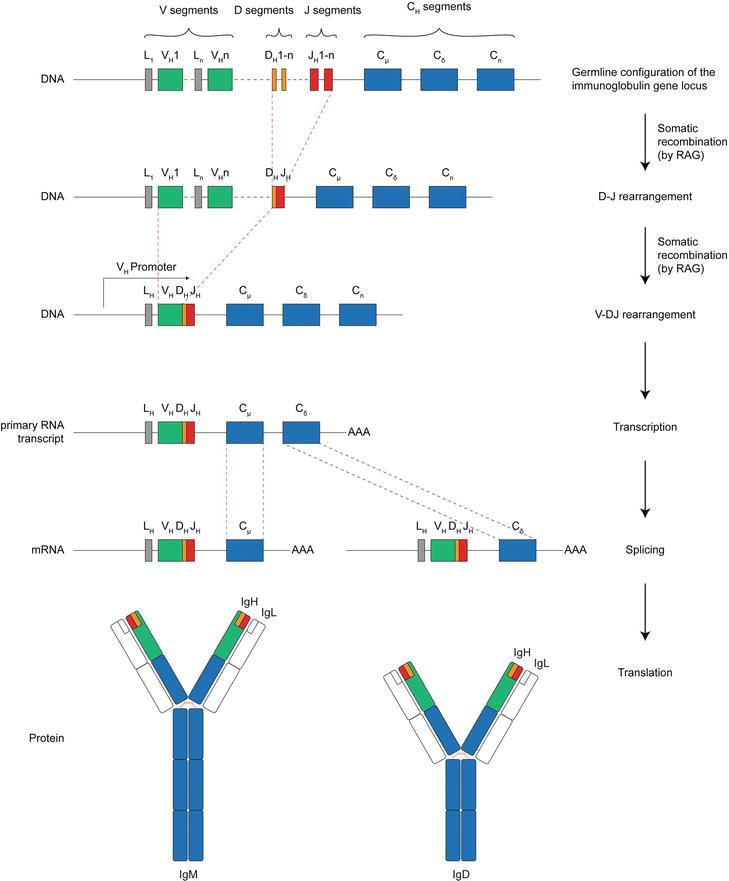 Figure 1: Variant regions of heavy and light chains formed through V(D)J recombination
Figure 1: Variant regions of heavy and light chains formed through V(D)J recombinationIn a single B cell, a diverse array of potential heavy chain (HC) and light chain (LC) pairs can undergo recombination and collectively generate. Considering that both HC and LC chains serve as antigen-binding sites, defining antigen specificity, more than 3 million unique antibodies can be produced. The diversity of antigen-binding sites combinations is achieved through the joining of various segment combinations and pairing of HC and LC chains.
Somatic Hypermutation
The process of somatic hypermutation (SHM) provides an additional source of antibody diversity following V(D)J recombination. The point mutations induced by SHM increase the variability of the variable region, occurring at a rate a million times higher than mutations in other genes. The introduction of double-strand breaks in the variable region triggers a DNA damage response, promoting error-prone repair processes, and consequently generating mutant antibodies.
SHM prompts a progressive enhancement of an antibody's affinity for antigens, a process termed affinity maturation (as illustrated in Figure 4). Some variant forms of immunoglobulins possess stronger antigen-binding capabilities than others, while certain variants can lead to nonproductive rearrangements. Mutations in the variable domain are typically favored as they neither augment antigen-binding nor alter the fundamental structure of the antibody. Mutations that enhance antigen-binding are often concentrated in the Complementarity Determining Region (CDR). In germinal centers, hypermutated B cells undergo a process of selection where they compete for various signals in an affinity-dependent manner, thereby outcompeting lower-affinity B cell clones. High affinity binders are selected, proliferate, and mature into antibody-secreting cells while lower-affinity clones are eliminated through apoptosis.
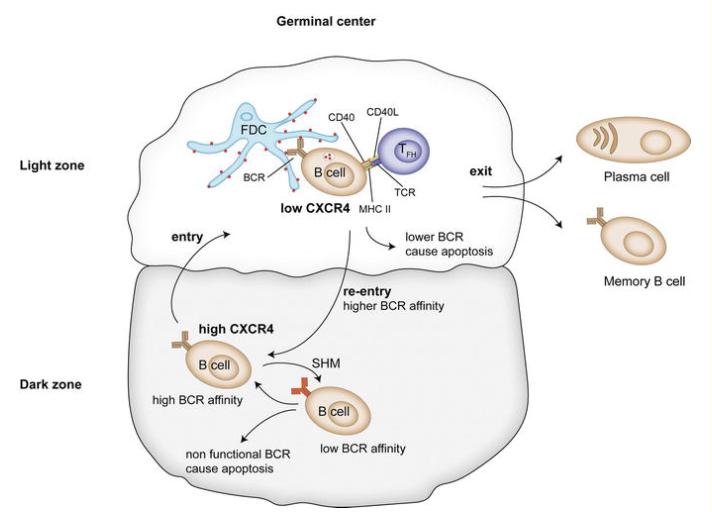 Figure 4 The affinity of the BCR is further increased by additional rounds of SHM
Figure 4 The affinity of the BCR is further increased by additional rounds of SHMClass Switch Recombination
In the course of B cell development, a cellular switch between the generation of one type of antibody and another takes place, a process referred to as class switch recombination (CSR). IgM is the initial immunoglobulin synthesized by B cells, where it embeds into the plasma membrane as a B cell receptor (BCR). Upon departure from the bone marrow, the cells commence the synthesis of membrane-bound IgM and IgD molecules. When these B cells interact and bind to an antigen via the BCR, this catalyzes the transformation of membrane-bound IgM to soluble IgM. As the immune response progresses, a class switch occurs, resulting in the production of IgG, IgE, or IgA antibodies. This process fosters secondary immune responses through memory B cells.
The class of an antibody is determined by the constant region of the HC, therefore, class switch recombination (CSR) takes place via the intrachromosomal deletion of switch (S) regions within the HC constant zone. Unlike V(D)J recombination, CSR transpires following antigen stimulation, is activated by helper T-cells, and involves different enzymes recognizing diverse flank sequences on DNA. Importantly, CSR only modifies the constant region without affecting the antigen-binding site. Consequently, CSR distributes the antigen-binding sites across various antibody classes, thereby introducing diversity to antibody immune responses and biological functions.
References
- Briney B, Inderbitzin A, Joyce C, et al. Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature, 2019, 566(7744): 393-397.
- Janeway Jr C A, Travers P, Walport M, et al. The generation of diversity in immunoglobulins//Immunobiology: The Immune System in Health and Disease. 5th edition. Garland Science, 2001.
- Wang F, Ekiert D C, Ahmad I, et al. Reshaping antibody diversity[J]. Cell, 2013, 153(6): 1379-1393.
- Methot S P, Di Noia J M. Molecular mechanisms of somatic hypermutation and class switch recombination. Advances in immunology, 2017, 133: 37-87.
- Backhaus O (2018) Generation of Antibody Diversity. Antibody Engineering. InTech. Available at: http://dx.doi.org/10.5772/intechopen.72818.