Fc-Fusion has diverse structures and complex glycosylation modifications. Therefore, in order to reduce the complexity of the sample, different methods are usually used for glycoprotein analysis according to protein size: intact and subunit protein level (Top and Middle-up), glycopeptide (Bottom-up) and free glycan level. Typically, methods used to characterize glycosylation modifications include lectin microarrays, reversed-phase liquid chromatography (RPLC), hydrophilic interaction chromatography (HILIC), anion exchange chromatography (AEX), porous graphitic carbon (PGC) or Capillary electrophoresis (CE), usually combined with techniques such as matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS), electrospray ionization mass spectrometry (ESI-MS), amperometric or fluorescence detection (FD), etc.
However, the glycosylation modifications of Fc-Fusion tend to be more complex and diverse than those of common mAb, with multiple N- or O-glycosylation sites, so techniques that are traditionally applied to mAb are not necessarily suitable for analyzing and characterizing Fc-Fusion.
HILIC is the most commonly used technique for glycan analysis in Fc-Fusion process development and quality control, which usually requires pre-separation of free N-glycans from Fc-Fusion and then fluorescence labeling before analysis. The procedure is as follows: first, the N-glycan is separated from the glycoprotein using enzymes such as PNGase F; then the N-glycan is fluorescently labeled by reductive amination with 2-aminobenzamide (2-AB); subsequently, the labeled glycan is separated using a HILIC column and identified according to the gradient of 2-AB-labeled dextran. However, HILIC (2-AB) also has some disadvantages. For example: the sample preparation time is long (∼40 h); it is prone to co-elution. The combination of HILIC-FD detection and MS identification techniques with RapiFluor MS labeling method can overcome these drawbacks, not only by significantly reducing the sample preparation time (∼1 h), but also by significantly improving the sensitivity of fluorescence and mass spectrometry detection. Moreover, the addition of MS detection provides a fully orthogonal identification method for FD, resulting in more reliable characterization and quantification in the case of co-elution.
Sialylation
Similarly, similar to the methods described above, the sialic acid modification of Fc-Fusion can also be characterized using methods to separate and label glycans. In general, N-acetylneuraminic acid and non-human N-glycylneuraminic acid can be used to introduce a negative charge into the target Fc-Fusion molecule and then analyze the sialic acid using AEX chromatography, which separates neutral glycans (non-sialylated) from monosialylated, disialylated and tri-sialylated glycans. It is important to note that these techniques cannot distinguish between different types of sialic acids, e.g. Neu5Ac and Neu5Gc. Due to the immunogenicity of the non-human naturally Neu5Gc, another complementary approach was required to characterize the sialylation of Fc-Fusion. The steps are: first, chemical separation of sialic acid from glycans; then, fluorescent labeling using DMB; and finally, RPLC chromatography and FD detection.
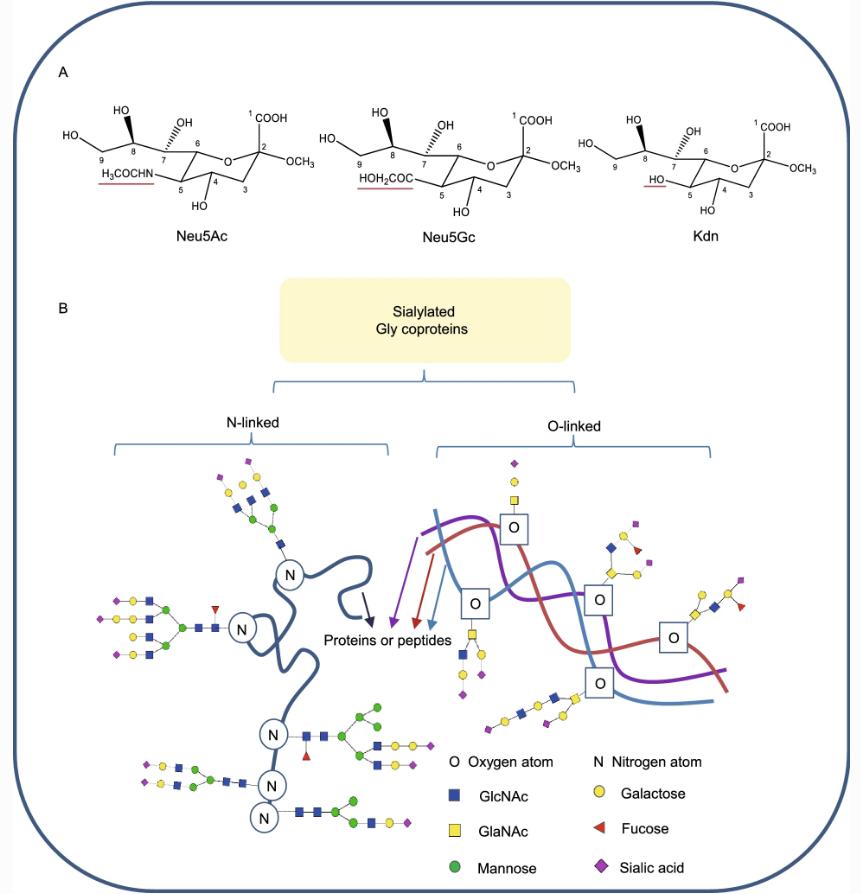
Structures of sialic acids and the diversity of sialylated glycoproteins.
O-glycosylation
Fc-Fusion usually carries multiple O-glycosylation sites in the non-IgG domain, and since it cannot be cleaved with PNGase F, different analytical methods are required for characterization. Typically, O-glycans are isolated by reductive β-elimination and then analyzed by PGC-ESI-MS or MALDI-TOF. Compared with N-glycans, O-glycan core structures are complex and diverse, which makes the characterization of O-glycan structures very challenging. However, since O-glycans can be randomly attached to the hydroxyl groups of amino acids such as serine and threonine, there may be a large number of O-glycosylation sites in the Fc-Fusion molecule. Therefore, obtaining specific information on glycan attachment sites is important for accurate analysis of glycan profiles.
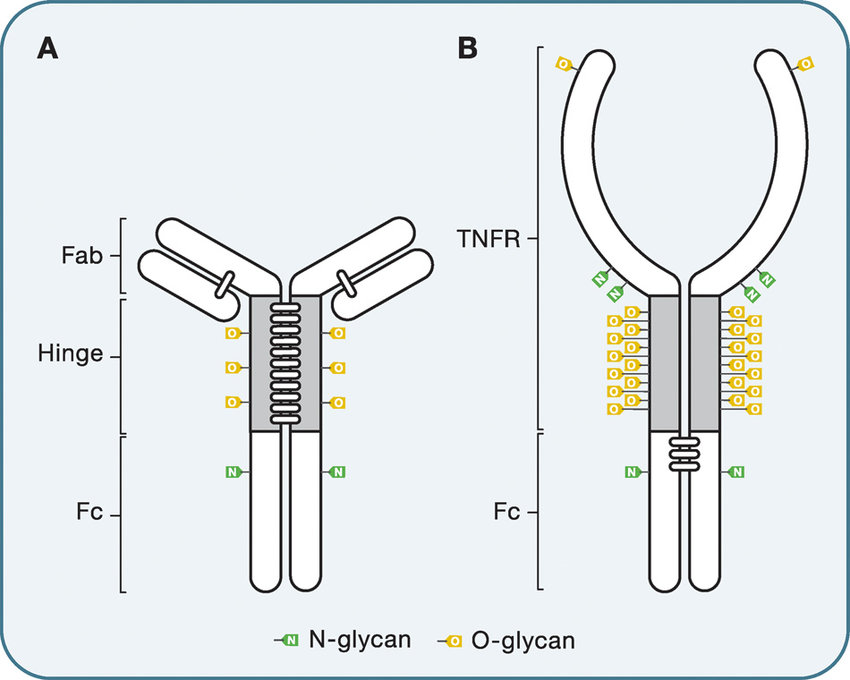
O-glycosylation of immunoglobulin G and Ig-fusion protein
Glycosylation Site Specificity
In general, glycan site-specific characteristics can provide important information on the safety and efficacy of Fc fusion products containing various N- and O-glycosylation sites, and site-specificity of glycosylation is essential for further development of the clinical potential of therapeutic products and for better understanding of CQA.
However, the above-described "separation-and-analyze " glycan analysis method results in the loss of site-specific information that can be obtained with peptide mapping techniques. Specifically: first, glycoproteins are digested using proteases such as trypsin to produce peptides and glycopeptides of about 0.5-5 kDa; then, the obtained peptides are analyzed using methods such as MALDI-MS or ESI-MS to obtain glycan profiles based on accurate mass information. In order to overcome the ion suppression effect of conventional peptides and high-abundance glycopeptides, chromatography such as RPLC, HILIC or electrophoretic separation techniques such as CE are usually used before MS detection to ensure the reliability of low-abundance carbohydrate characterization. In addition, tandem MS analysis of glycopeptides (eg, electrotransfer dissociation) can be used to identify glycan sites and determine site occupancy. Usually, O-glycan sites do not have a sequence similar to that of N-glycans, so O-glycans may be randomly distributed throughout the protein molecule. Therefore, this glycan site specificity and its occupancy information is very valuable for Fc-Fusion containing O-glycans. A distinct advantage of the Middle-up method compared to the Bottom-up method is that it can obtain important site-specific glycan information with fewer sample preparation steps. Furthermore, fully automated analysis of Fc-Fusion glycans at the Middle-up level is possible using the emerging 3D-LC/MS method. Therefore, the Middle-up method provides a very convenient option for routine analysis or rapid batch-to-batch comparison of the glycosylation profiles of therapeutic proteins. Overall, N- and O-glycan characterization techniques for Fc-Fusion are diverse. However, special attention still needs to be paid to the modification sites of polyglycosylation, the site-specific glycosylation characteristics, and the occupancy of N- and O-glycans at the sites. Therefore, it is necessary to combine multiple orthogonal techniques to analyze Fc-Fusion to obtain comprehensive and efficient complex glycosylation information.
Conclusion
The glycosylation of Fc-Fusion has important biological significance, which may change its immune effector function by changing the binding affinity of the target molecule to Fcγ receptors, and may also affect the PK/PD and biosafety of the molecule. Therefore, in-depth understanding and and monitoring of the glycosylation of Fc-Fusion by various characterization techniques are needed.
Since the glycosylation of Fc-Fusion is extremely complex, involving multiple N- and O-glycan sites and a large number of sialic acids, complementary new analytical methods are required for accurate characterization. To cope with the extremely complex features of Fc-Fusion, more robust characterization methods such as multidimensional LC or ion mobility spectroscopy-mass spectrometry (IM-MS) will need to be developed in the future. Furthermore, due to the large amount of sialic acid present in its structure, bio-inert chromatography systems and bio-inert chromatography columns can be employed to limit unnecessary adsorption in glycan analysis. Furthermore, due to the very high number of charge heterogeneities and sialic acids in Fc-Fusion, the usual AEX characterization of Fc-Fusion is often not as powerful as it could be, and MS-compatible analytical steps must be developed in AEX to ensure that a sufficient amount of information can be obtained from such analysis to meet regulatory agency requirements.
References
- Li, F., Ding, J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell 10, 550–565 (2019).
- Jonathan Sjögren, Rolf Lood. On enzymatic remodeling of IgG glycosylation; unique tools with broad applications. Glycobiology 30(4) (2019).
Related Service
Fusion Proteins Characterization
Monoclonal Antibody Characterization
Antibody Drug Characterization
Antibody Drug Conjugates (ADCs) Characterization
Bispecific Antibody Characterization
Related Resource
Introduction and Characterization of Monoclonal Antibody Drugs
Application of Mass Spectrometry Technology in Antibody Drug Analysis
Characterization of Monoclonal Antibodies in Biopharmaceuticals






