What is fluorescence?
Fluorescence is a kind of light-induced cold luminescence phenomenon. When irradiated by incident light of a certain wavelength (usually ultraviolet or X-ray) at room temperature, a substance absorbs the light energy, enters an excited state, and immediately deexcites and emits an outgoing light (usually in the visible wavelength) longer than the incident light. Once many fluorescent substances stop the incident light, the luminescence phenomenon will disappear immediately. The emitted light with this property is called fluorescence.
Principle of fluorescence:
When light irradiates certain atoms, the energy of the light causes some electrons around the nucleus to transition from the original orbit to a higher energy orbit, that is, transition from the ground state to the first excited singlet state or the second excited singlet state. Electrons at both states are unstable, so they will return to the ground state. And when they do, the energy is released in the form of light, generating fluorescence.
What is fluorescence spectroscopy?
Any fluorescent compound has two characteristic spectra: excitation spectrum and emission spectrum. The excitation spectrum reflects the dependence of the measured fluorescence intensity on the excitation wavelength at a fixed emission wavelength. The emission spectrum reflects the wavelength distribution of fluorescence measured at a certain excitation wavelength.
Absorption spectrum: the curve of absorption intensity and incident wavelength of the compound. It reflects the permissible transition between the ground state energy level and the excited state energy level. The apparent color of a substance in its usual state depends largely on its absorption properties.
Excitation spectrum: fixed emission wavelength, the curve of the relationship between the emitted light intensity (fluorescence/phosphorescence) of the scanned compound and the incident light wavelength. It reflects the transition between the ground state and all energy levels related to the fluorescence emission. The relationship presented is more selective, but sometimes less direct, than the absorption spectrum.
Emission spectrum: fixed excitation wavelength, the curve of the relationship between the emitted light intensity (fluorescence/phosphorescence) of the compound and the wavelength of the incident light is scanned. It is the reverse process of light absorption, also known as fluorescence spectroscopy.
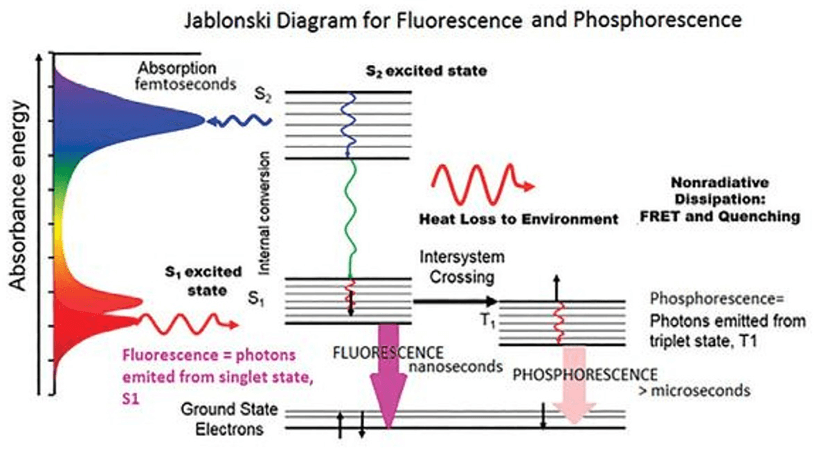
The Jablonski Diagram of molecular absorbance and fluorescence (Jablonski, 1933)
Fluorescence spectrum can provide information such as excitation spectrum, emission spectrum, peak position, peak intensity, quantum yield, fluorescence lifetime, fluorescence polarization degree, etc., and is the basis of qualitative and quantitative fluorescence analysis.
Features of fluorescence spectroscopy:
(1) Stokes shift. The difference in the wavelength between the excitation spectrum and the emission spectrum, and the emission spectrum wavelength is longer than the excitation spectrum wavelength.
(2) The shape of the emission spectrum has nothing to do with the excitation wavelength.
(3) Mirroring rules. The fluorescence emission spectrum has a mirror symmetry relationship with its absorption spectrum.






