Any drug has a unique structure that affects its function. For example, a variety of kinases can only be activated when exposed to phosphorylation sites, thereby regulating a range of reactions in the body; the opening of integrin in the head increases the adhesion of the leukocyte cells, while the closure does not. The study of drug structure is the basis of drug research to solve existing diseases and provide the basis for the development of new drugs. The protein biosimilar also has its unique structure, which plays a fundamental role in the study of its function.
Protein biosimilars are essential proteins. Protein has a specific conformation, and its structure is divided into four levels, including primary structure, secondary structure, tertiary structure and quaternary structure. The secondary, tertiary and quaternary structures are generally referred to as three-dimensional conformations or advanced structures.
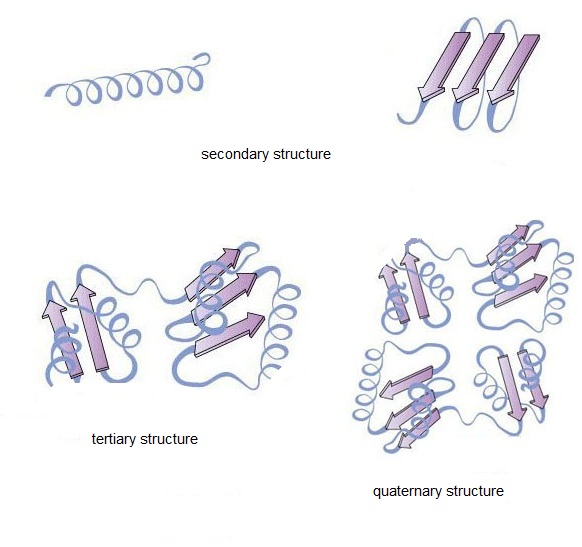
Figure 1. Four structural levels of the protein
Structural Characterization at Creative Proteomics
The primary structure determines the advanced structure. The primary structure of protein biosimilar is formed by various amino acids through the peptide bond in a certain order. Studying the type and sequence of amino acids is much meaningful for studying the structure of protein biosimilars, and Creative Proteomics can provide sufficient information in this regard.
- Amino Acid Composition
Amino acid composition analysis is a classical method for protein analysis. It can be used to determine the composition and content of amino acids in proteins and peptides as well as the contents of non-typical amino acids. Amino acid analysis is a necessary step before completing sequencing of biopharmaceuticals. At Creative Proteomics, amino acid analyzer and reversed-phase high-performance liquid chromatograph (UPLC) and other instruments are available to detect amino acids.
- Amino Acid Sequence
Proteins and polypeptides are formed by a variety of amino acids connected by peptide bond in a certain order. Different amino acids of proteins are arranged in different order, which is one of the reasons for protein diversity. Based on advanced instruments, we can provide different methods for amino acid sequence, including Edman method, peptide mapping fingerprinting (PMF) and de novo sequencing.
- N/C-terminal Sequencing
The determination of N/C terminal is particularly important for understanding the sequence of amino acids. Our N-terminal sequencing platform containing Edman degradation and mass spectrometry-based methods can help you choose the suitable methods and obtain reliable results. The most common way to determine the C-terminal is to add carboxypeptidase to the protein solution, take regular samples, and determine the terminal amino acids by analyzing the curve of the amino acid concentration over time.
- Peptide Mapping
Peptide mapping is a widely used tool for protein identification and protein modification. It is usually performed by reversed-phase liquid chromatography, and mainly used for the confirmation of primary protein structure.
- Disulfide bond Analysis
Disulfide bond is a common post-translational modification of proteins, which can stabilize the spatial structure of proteins and maintain and regulate their biological activities. Therefore, determining the location of disulfide bonds in proteins is an important aspect to fully understand the chemical structure of disulfide bond proteins. We can provide diagonal electrophoresis to locate disulfide bonds.
- Glycosylation Analysis
N-glycosylation and O-glycosylation are two main types of glycosylation of proteins. The determination methods of glycoprotein identification and glycosylation sites include PNGase method and Endo method.
Our Advantages
- We contain a large amount of accurate information about the structure of protein biosimilars to meet your needs at any time.
- We can provide various experimental methods and technologies to detect the primary structure and the advanced structure of protein biosimilars. On the one hand, we have some methods, such as automatic amino acid analyzer, sanger method, enzyme degradation method; on the other hand, we have various technologies, such as X-ray crystal diffraction analysis, three dimensional reconstruction technique and nuclear magnetic resonance technique, etc.
Creative Proteomics provides comprehensive structural characterization for biosimilars. In addition, our service can be applied to other drugs, such as sugar drugs, polypeptide drugs and so on. If you have any questions or specific requirements, please feel free to contact us.
References
- Wishart D S. Characterization of biopharmaceuticals by NMR spectroscopy. Trends in Analytical Chemistry, 2013, 48(1):96-111.
- Richardson J S. The anatomy and taxonomy of protein structure. Adv Protein Chem, 1981, 34(34):167-339.
3. Heiger D, Grimm R; etc. Peptide mapping and analysis using capillary electrophoresis. Agilent Technologies, 1993.






