Quality Control Essentials and Analytical Advances for Monoclonal Antibody Drugs
Monoclonal antibody (mAb) drugs have become a cornerstone of modern biopharmaceutical research and development. Over the past decade, their innovation and production have accelerated, positioning them at the centre of global competition in the life sciences. However, their manufacturing processes differ significantly from those of traditional small-molecule drugs, creating unique challenges for quality control and testing.
Unlike chemical drugs, monoclonal antibodies are large, complex proteins produced in living cells, meaning their purity, stability, and consistency depend heavily on how they are manufactured. For developers and contract research organisations (CROs), understanding the critical control points in mAb production is key to meeting strict regulatory expectations and ensuring patient safety.
Distinctive Quality Control Considerations
- Biological complexity: mAbs exhibit structural variations such as glycosylation patterns, which must be tightly monitored.
- Process-driven impurities: Residual host cell proteins (HCPs), DNA fragments, and culture by-products require rigorous removal.
- Product-related issues: Aggregates or charge variants can impact therapeutic performance and immunogenicity.
Analytical Tools Supporting mAb Quality
The shift toward biologics has spurred the adoption of advanced analytical technologies to detect and quantify impurities more precisely:
- Capillary Electrophoresis (CE): Enables sensitive profiling of charge variants and fragmented forms.
- High-Performance Liquid Chromatography (HPLC): Essential for separating and characterising molecular impurities.
- Emerging methods: Hybrid approaches combining mass spectrometry and chromatography are gaining traction for deeper molecular insights.
Regulatory Alignment and Future Directions
Regulators, including the FDA and EMA, demand robust quality frameworks for biologics. New monoclonal antibody approvals increasingly reference advanced analytical standards, setting benchmarks for industry best practice. Looking forward, cutting-edge tools such as multi-dimensional chromatography and real-time monitoring systems are expected to become standard in mAb production workflows.
For biopharma innovators, adopting these analytical strategies early offers a competitive edge—shortening development timelines and supporting faster approvals.
Monoclonal Antibody Drugs: Structure, Development, and Quality Control Challenges
Monoclonal antibody (mAb) drugs are large protein therapeutics built on an immunoglobulin G (IgG) framework. For more on stability risks, see Protein Aggregation: In-Depth Analysis of the Causes and Influencing Factors.Each molecule consists of two heavy and two light chains forming antigen-binding regions that recognise highly specific targets. This structural precision underpins their effectiveness in treating a broad range of diseases.
With advances in biotechnology, mAb production methods have matured, and their role in global healthcare has grown significantly. Leading pharmaceutical companies—particularly international biopharma giants—are heavily investing in mAb research and manufacturing. Currently, the U.S. Food and Drug Administration (FDA) has approved more than 60 monoclonal antibody drugs, and over 300 others are in clinical trials. These therapies address diverse conditions, including cancer, autoimmune disorders, cardiovascular disease, and infectious illnesses.
Expanding Classes of Monoclonal Antibody Therapies
Beyond established drugs like adalimumab, bevacizumab, trastuzumab, and widely used PD-1/PD-L1 inhibitors, innovation is accelerating:
- Antibody-drug conjugates (ADCs): Targeted delivery of cytotoxic payloads to diseased cells.
- Bispecific antibodies: Molecules designed to bind two different antigens simultaneously.
- Immune checkpoint inhibitors: Agents unlocking immune responses against tumours.
These next-generation formats represent some of the most competitive areas in global biologics pipelines.
Quality Control: A Distinct Challenge
Unlike traditional chemical drugs, mAbs are produced in living systems and carry unique impurities absent in small-molecule processes. This complexity introduces quality risks and requires multi-dimensional analysis. Robust impurity profiling is essential to confirm each drug's safety, efficacy, and consistency across batches.
For developers and CRO partners, mastering these quality control strategies—particularly impurity analysis—remains critical for regulatory compliance and maintaining trust in mAb therapies.
You may interested in
1. Quality Standards for Monoclonal Antibody Drugs
The current regulatory standards for monoclonal antibody (mAb) drugs are primarily based on guidelines set by the World Health Organization (WHO), the International Conference on Harmonization (ICH), and regulatory bodies such as the FDA and the European Medicines Agency (EMA). These standards apply to biotechnological products, biopharmaceuticals, and biosimilars. The guidelines ensure that mAb drugs meet international technical requirements for registration and quality control.
2. Monoclonal Antibody Drug Testing
2.1 Testing for Physical and Chemical Properties
Monoclonal antibodies are produced through cell expression, followed by purification and formulation processes. Various factors, such as the formulation process, storage conditions, and transport methods, can influence the formation or disruption of both covalent and non-covalent bonds in the drug. These factors can ultimately alter the drug's physical and chemical properties. Therefore, testing these properties is an essential part of quality control. Key tests include assessments of appearance, reconstitution time, dose uniformity, pH levels, visible contaminants, clarity, insoluble particles, osmolality, and moisture content.
2.2 Control of Impurities and Analytical Techniques
Capillary Electrophoresis (CE) for Purity and Impurity Analysis
Capillary electrophoresis (CE) is an advanced technique that utilizes elastic quartz capillaries as the separation channel and a high-voltage direct current field as the driving force. The technique separates molecules based on their charge and molecular weight, combining features of both electrophoresis and chromatography. Monoclonal antibody (mAb) drugs, being heterogeneous in charge and molecular weight, require careful purity control throughout production and batch release to ensure they remain intact and free from unwanted modifications.
CE is essential for the purity and impurity analysis of mAb drugs due to its speed, sensitivity, and reliability. Among the various CE modes, the three most commonly used in monoclonal antibody analysis are:
- Imaged Capillary Isoelectric Focusing (iCIEF): This mode separates molecules based on their isoelectric points (pI). A pH gradient is formed in the electrolyte solution, and each component migrates to its respective pI, forming bands. iCIEF provides detailed information on protein charge heterogeneity and is widely used for identifying isoelectric points and checking charge variants. Compared to traditional gel electrophoresis, iCIEF offers faster results, requires less sample, and delivers higher resolution and better reproducibility.
- Capillary Zone Electrophoresis (CZE): In CZE, components move through a detector according to their electrophoretic mobility and electro-osmotic flow, based on their charge-to-mass ratio. This technique is particularly useful for checking charge heterogeneity in mAbs. Compared to iCIEF, CZE is faster and allows for lower UV detection at 214 nm, providing higher sensitivity. CZE can also be coupled with mass spectrometry to analyze the structure of charge variants, providing valuable structural insights.
- Capillary Gel Electrophoresis (CGE): This technique is used for analyzing molecular size heterogeneity (reduced and non-reduced forms) and N-glycan profiling. When monoclonal antibodies interact with sodium dodecyl sulfate (SDS), they form complexes that are separated using CE, often referred to as SDS-free capillary electrophoresis (CE-SDS). CE-SDS offers faster, more reliable results than traditional SDS-PAGE, with higher precision and resolution. It also eliminates tailing peaks and sample residue, making it more accurate, stable, and objective.
Glycan and Host Cell DNA Analysis
Recent advancements in CE have enabled the exploration of glycan analysis in monoclonal antibodies. N-glycans are enzymatically cleaved and labeled, then analyzed using CE with laser-induced fluorescence detection (CE-LIF). This approach significantly reduces the analysis time compared to traditional liquid-phase methods and provides faster, simpler results.
Additionally, CE-LIF is used for detecting host cell DNA (HCD) residues in mAbs. This technique offers superior sensitivity compared to ELISA, with detection limits as low as 6.25 ng/uL. The analysis is highly reproducible, with low variability in migration times and peak areas. CE-LIF provides enhanced separation compared to agarose gel electrophoresis, enabling more accurate identification of HCD.
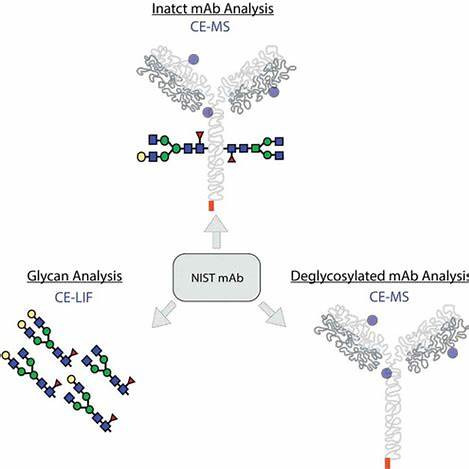 Figure 2. CE-MS and CE-LIF characterization of intact monoclonal antibody proteoforms and glycoforms
Figure 2. CE-MS and CE-LIF characterization of intact monoclonal antibody proteoforms and glycoformsHigh-Performance Liquid Chromatography (HPLC)
High-performance liquid chromatography (HPLC) is another widely used method for purity and impurity analysis. To understand structural stability further, you can read Disulfide Bond Structure, Function, Application, and Control Strategies.Among the various types of HPLC, reverse-phase HPLC (RP-HPLC), size-exclusion chromatography (SEC-HPLC), and ion-exchange chromatography (IEC-HPLC) are the most common for mAb quality control, each addressing different aspects of the drug's purity and composition.
RP-HPLC for Peptide Mapping in Monoclonal Antibodies
Reverse-phase high-performance liquid chromatography (RP-HPLC) is commonly used for peptide mapping in monoclonal antibody (mAb) drugs. This technique involves the digestion of mAb drugs using trypsin to form peptide fragments, which are then analyzed by RP-HPLC to assess the integrity and accuracy of the primary structure. Peptide mapping serves as a "fingerprint" of the protein and its enzymatic products, making it an essential quality control step for mAb drug release. It can detect a range of modifications, such as point mutations, oxidation, deamidation, N-terminal cyclization, C-terminal lysine processing, and N-glycosylation, among other unexpected expression variants.
The peptide mapping process includes four main steps: protein purification, selective enzymatic digestion, chromatographic separation of peptides, and peptide analysis and identification. For effective analysis, the method must provide enough peptide fragments and as high a coverage as possible. However, the method does not include steps for reducing disulfide bonds to their reduced free thiol states, which can result in incomplete cleavage peaks when using trypsin. For RP-HPLC with UV detection, achieving a sufficiently large separation window is crucial to resolve all component peaks. Using small-particle, long columns and ultra-performance liquid chromatography (UPLC) systems can significantly improve separation efficiency and speed.
SEC-HPLC for Polymer Detection
Size-exclusion chromatography (SEC-HPLC) is used to separate proteins by their molecular size, including oligomers, dimers, monomers, and certain fragments that may appear during the process. SEC-HPLC is widely used for detecting mAb drug aggregates and remains one of the most common methods for quality control and release testing. It offers good resolution for separating dimers and aggregates, has a relatively short analysis time, and is easy to operate. The collected fractions can be analyzed further if needed. In SEC-HPLC separations, the sample passes through the column with isocratic elution, so the volume of the sample injection system, tubing, and detector affects the separation. Generally, smaller system volumes lead to narrower peaks and better separation. Using smaller-particle (3 µm) columns can achieve higher flow rates without sacrificing separation efficiency.
IEC-HPLC for Charge Variants
Ion-exchange chromatography (IEC-HPLC) is another method for assessing charge heterogeneity in mAbs. Compared to capillary electrophoresis (CE), IEC-HPLC is simpler to operate and detects differences in protein surface charge distributions. Weak cation-exchange columns are commonly used to detect acidic charge isoforms (which elute before the main peak) and basic charge isoforms (which elute later). These charge variants are often products of glycosylation and deamidation but have limited resolution, making IEC-HPLC less effective for precise impurity separation. However, as mass spectrometry (MS) techniques advance, liquid chromatography-mass spectrometry (LC-MS) is increasingly applied to mAb quality control. High-resolution mass spectrometry, such as quadrupole-time-of-flight (QToF) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF-MS), offer significant advantages in protein, peptide mapping, glycan analysis, protein quantification, and sequencing.
Table: Key Analytical Methods for Monoclonal Antibody Drugs
| Analytical Method | Main Application | Advantages | Limitations |
|---|---|---|---|
| Complete Protein Analysis | Whole molecule weight analysis, light chain quantification | Provides aggregate and quality information | Requires experienced personnel; not a routine QC method |
| Peptide Mapping | Detects glycosylation, oxidation, deamidation, disulfide bonds, etc. | Offers detailed quality and structural confirmation | Technically demanding, time-consuming, not routine QC |
| Glycan Analysis | Profiles glycan composition and structural heterogeneity | No derivatization needed; precise glycan insights | Sample prep can be long; harder for routine testing |
| Protein Impurity Testing | Detects host cell proteins (HCP), host cell DNA, and protein metabolites | High sensitivity and reliability | May require method adjustment to meet regulations |
| Protein Sequencing | Confirms N-terminal and C-terminal amino acid sequences | Accurate sequence determination | Not typically used as a QC method |
HILIC-HPLC for Glycan and Surfactant Analysis
Hydrophilic interaction liquid chromatography (HILIC-HPLC), often coupled with fluorescence detectors (FLD) or corona-charged aerosol detection (CAD), plays a vital role in the analysis of oligosaccharides and surfactants used in mAb drug formulations. HILIC-HPLC is particularly useful for detecting oligosaccharide chains and excipients, such as surfactants, that are introduced during the manufacturing process.
Advanced Multi-Dimensional Chromatography for Impurity Detection
As multidimensional chromatography techniques evolve, two-dimensional high-performance liquid chromatography (2D-HPLC) is increasingly used to separate or enrich difficult-to-resolve impurities, which can then be analyzed with mass spectrometry (MS), infrared (IR), or nuclear magnetic resonance (NMR) spectroscopy. This advancement has significantly improved impurity separation and identification in mAb drugs.
Cell Residue Impurity Analysis
In addition to routine sterility, abnormal toxicity, endotoxin, and pyrogen tests, mAb drugs must undergo strict controls for host cell protein (HCP) and DNA impurities introduced during the production process. For removal techniques, see Strategies for Host Cell Protein (HCP) Clearance.These impurities can affect the drug's potency and potentially cause immune responses, resulting in allergic reactions, toxicity, immunogenicity, or carcinogenicity. The FDA recommends using highly sensitive methods for detecting HCP impurities, which should remain below detection limits (usually <100 ppm). For CHO cell-derived mAbs, HCP levels should be less than 0.05% of total protein content. While international standards for HCP limits are still under development, enzyme-linked immunosorbent assays (ELISA) remain the most commonly used method for detecting HCP and Protein A residues in mAbs, as recommended by the General Principles for Biologics Quality Control Analysis Method Validation.
Regulatory Requirements for DNA Residues
WHO and regulatory agencies worldwide require that residual DNA from CHO host cells in mAb drugs should not exceed 10 pg. Quantitative PCR (q-PCR) is considered the most suitable method for detecting DNA residues, as outlined by the United States Pharmacopeia (USP). Although China's standards for DNA residue detection are still evolving, q-PCR is commonly used in mAb production facilities.
Quality by Design (QbD) in mAb Impurity Analysis
With the advancement of mAb research and analysis technologies, the "Quality by Design" (QbD) concept has become increasingly important in mAb impurity analysis. The trend is moving toward automation, higher specificity, improved sensitivity, miniaturization, and high-throughput real-time analysis. Technologies such as Lab-on-a-Chip (LOC) for impurity residue detection, automated protein purification, digestion, desalting, peptide fractionation, and glycan enrichment are all becoming more mature. High-resolution mass spectrometry (HRMS) for both qualitative and quantitative analysis is also gaining traction in process development and batch release. These innovations are driving the adoption of new techniques and methods in mAb impurity analysis, ultimately improving drug efficacy, safety, and consistency.
References
- Suprit Deol, Yutaka Matsuda & Yuki Hiruta, Current advances in separation chemistry for antibody purification and analysis, Analytical Sciences, 2025, 41, 653–666
- Mansi Shah et al., Analytical Techniques for the Characterization and Quantification of Monoclonal Antibodies, Pharmaceuticals, 2024, 16(2): 291.
- B. Nunnally et al., A Series of Collaborations between Various Pharmaceutical Companies and Regulatory Authorities Concerning the Analysis of Biomolecules Using Capillary Electrophoresis, Chromatographia, 2006, 64(5-6), 359-368.
- Meriem Dadouch, Yoann Ladner & Catherine Perrin, Analysis of Monoclonal Antibodies by Capillary Electrophoresis: Sample Preparation, Separation, and Detection, Separations, 2021, 8(1): 4.
- Mansi Shah et al., Capillary Electrophoresis Methods for Impurity Profiling of Drugs: A Review of the Past Decade, Journal of Pharmaceutical Analysis, 2022, 12(1): 15-28.
- Quan Liu et al., Rapid Identification of Antibody Impurities in Size-Based Electrophoresis via CZE-MS Generated Spectral Library, Scientific Reports, 2024, 14(20239).

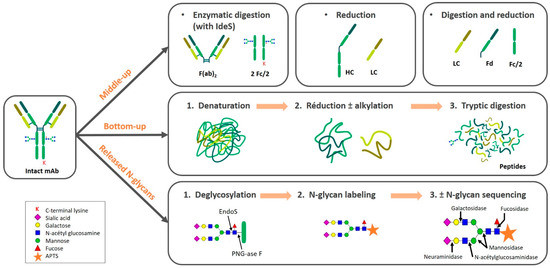 Figure 1.
Figure 1.