In recent decades, techniques such as Electrospray Ionization (ESI), Matrix-assisted Laser Desorption/Ionization (MALDI), and Surface-enhanced Laser Desorption/Ionization (SELDI) have prominently emerged in the field of mass spectrometry analysis. Mass analyzers encompass magnetic sector analyzers, quadrupole filters (Q), ion trap analyzers, ion cyclotron resonance (ICR) analyzers, and time-of-flight analyzers (TOF).
Within the realm of mass spectrometry analysis, the initial step involves ionization, wherein the analyte is processed to generate charged gas-phase ions. The two most efficacious methods for soft ionization in protein mass spectrometry are MALDI and ESI. These methodologies have become pivotal in elucidating the intricate molecular details of biological specimens, contributing significantly to the advancement of analytical methodologies in the life sciences.
This progression underscores the pivotal role that technological advancements play in elevating the precision and scope of mass spectrometry applications. The continual refinement and integration of ionization techniques with diverse mass analyzers underscore the dynamic nature of contemporary analytical methodologies in the field of biological mass spectrometry.
Select Service
What are ESI and MALDI
Electrospray Ionization (ESI):
Electrospray Ionization (ESI) stands as a soft ionization method rooted in electrospray technology. In ESI, charged droplets undergo evaporation and ionization under the influence of a high-pressure electric field. The ions present on the surface of the droplets are accelerated in the electric field, culminating in the formation of gas-phase ions. Notably, this process can yield multiply charged ions, facilitating the detection of high molecular weight substances.
MALDI:
Matrix-assisted Laser Desorption/Ionization (MALDI) represents a widely employed soft ionization technique for the analysis of large biomolecules. MALDI accomplishes ionization by irradiating the sample, desorbing it from the matrix, and subsequently ionizing it through laser excitation. When compared to ESI and APCI, MALDI proves particularly suited for the analysis of high molecular weight substances such as proteins and nucleic acids. The MALDI-TOF technique has made substantial strides in areas like microbial mass spectrometry, nucleic acid analysis, and imaging mass spectrometry, while ESI finds broad application in pharmaceutical enterprises, environmental monitoring, laboratories in hospitals, and educational institutions.
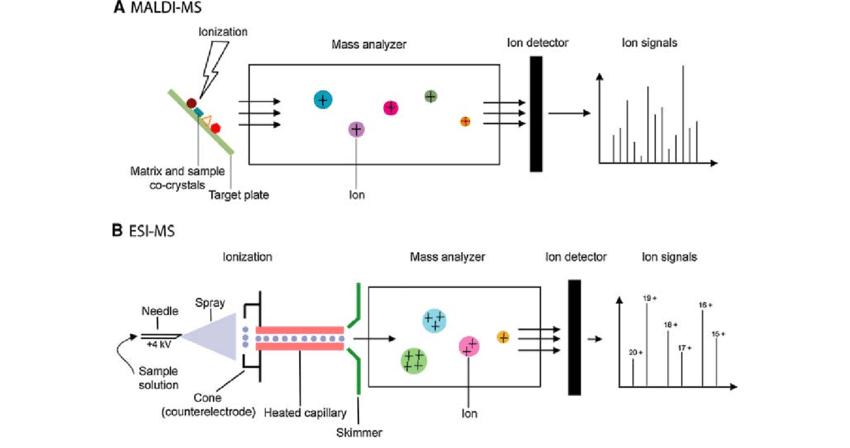 MALDI-MS and ESI-MS procedures (Ying Lin et al,. 2019)
MALDI-MS and ESI-MS procedures (Ying Lin et al,. 2019)Comparison of ESI and MALDI
However, there are certain distinctions between MALDI and ESI, particularly in the realm of large molecule detection, notably within the domain of proteins. The following constitutes a comprehensive comparative analysis of these two techniques:
MALDI Technology: Pros and Cons
| Pros | Cons |
| High Sensitivity: MALDI can detect samples at very low concentrations, making it advantageous for the analysis of trace samples. | Matrix Interference: Requires the use of matrix-assisted desorption and ionization, and the matrix may introduce interference peaks affecting the resolution of mass spectra. |
| High Resolution: The generated mass spectra exhibit high resolution, allowing clear distinction of different molecular ion peaks. | Poor Reproducibility: Experimental results may exhibit poor reproducibility, necessitating multiple experiments for reliable data. |
| Broad Applicability: Applicable to the analysis of various sample types, including large biomolecules, organic small molecules, and synthetic high-molecular-weight materials. | Poor Analysis of High Salt and High Buffer Samples: Ineffective analysis of samples containing high salt or high buffer, requiring additional sample processing steps. |
| Simple Sample Preparation: Compared to other mass spectrometry techniques, MALDI involves a relatively simple sample preparation process without complex chemical treatments. | High Instrument Cost: MALDI instruments are relatively expensive, with high maintenance costs, potentially limiting usage in some laboratories. |
| Compatibility with Multiple Mass Analyzers: Can be coupled with various mass analyzers, such as Time-of-Flight (TOF) and Ion Trap Mass Analyzers, expanding its application range. |
ESI Technology: Pros and Cons
| Pros | Cons |
| High Sensitivity: Offers high sensitivity for the analysis of trace samples. | Sample Preprocessing Required: Samples need preprocessing before electrospray, and the analysis detection time is relatively long. |
| High Resolution: Produces mass spectra with high resolution, suitable for protein and peptide analysis. | Poor Analysis of High Salt and High Buffer Samples: The analysis may be less effective for samples with high salt or high buffer, requiring special sample processing. |
| Applicable to Large Molecules: Generates multiply charged ions, expanding the detection range of mass spectra. | High Instrument Cost: The instrument design is complex, leading to higher costs. |
| Good Selectivity: Demonstrates good selectivity for protein and peptide analysis, with minimal background interference, facilitating the identification of target compounds. | Relatively Long Analysis Detection Time |
| Comparison of ESI and MALDI | MALDI | ESI |
| Charge | single | multiple |
| Sample preparation | solid | liquid |
| Speed | rapid | slow |
| capacity | large | small |
| MS/MS capability | weak | strong |
How to choose ESI and MALDI
When selecting an ion source, a comprehensive assessment must be made based on specific application requirements and experimental conditions. The following factors are pivotal considerations in the choice of an ion source:
Sample Characteristics: Different ion sources are suited for different sample properties. For instance, for samples with high polarity and large molecular weight, Electrospray Ionization (ESI) is more suitable.
Experimental Objectives: Distinct ion sources yield different types of ions, necessitating the selection of an ion source based on experimental objectives. For example, if determining the relative molecular mass of a compound is the goal, Matrix-assisted Laser Desorption/Ionization (MALDI) might be more appropriate.
Instrument Performance: Different ion sources require varied instruments, and the performance of the instrument influences the choice of the ion source. For instance, if the laboratory already possesses an Electrospray Mass Spectrometer, opting for Electrospray Ionization (ESI) may be more economical and convenient.
Ease of Operation: While ensuring experimental efficacy, choosing an ion source with straightforward operation can reduce experimental time and minimize errors.
Cost: Ion sources vary in price and operating costs, necessitating consideration of the laboratory's budget and long-term usage costs.
Quantitative Analysis of Large Molecules through MALDI and ESI
As widely recognized, biomacromolecules undergo ionization in the Electrospray Ionization (ESI) source, producing multiply charged ions and expanding the mass range detectable by the mass analyzer by several to tens of times. Consequently, ESI finds applications in the qualitative and quantitative analyses of peptides, proteins, and enzymatic protein fragments.
Functioning as a soft ionization technique, ESI minimally generates fragment ion peaks, providing an advantage in the quantitative analysis of large molecular substances. However, owing to its characteristic of producing multiply charged ions, deconvolution operations with software are necessary when determining molecular weights.
ESI has found widespread utility in the field of large molecule quantitative analysis, with classic applications encompassing insulin, antibody-drug conjugates (ADCs), hormones, and biopharmaceuticals, among others, although these are not exhaustively listed here.
On the other hand, employing the principle of matrix-assisted laser desorption/ionization (MALDI) where matrix absorbs energy upon laser irradiation to facilitate desorption, biomacromolecules in the MALDI source exhibit high ionization efficiency, typically yielding ions carrying a single charge. This process minimally induces polymer chain fragmentation, typically resulting in molecular ions and their complexes.
Traditional strengths of MALDI-Time-of-Flight Mass Spectrometry (MALDI-TOF) lie in microbial identification. Recent advancements in technologies such as nucleic acid mass spectrometry and mass spectrometry imaging primarily rely on its robust qualitative capabilities. It is noteworthy that cases have demonstrated MALDI's substantial potential in protein quantitation. Currently, MALDI has been applied to the quantitative analysis of peptides and proteins, including M proteins, Insulin-like Growth Factor 1 (IGF1), glycated hemoglobin, and serum anthrax lethal factor, with the analysis process also incorporating qualitative analysis of protein variants.
In summary, within the realm of protein qualitation and quantitation, MALDI, as a rapidly advancing technology, holds tremendous potential. Concurrently, given its high qualitative capabilities, MALDI and ESI together form a "twin-star" combination in laboratories, promising increased convenience for clinical applications.