Monoclonal antibodies (mAbs) are playing an increasingly important role in the development of new drugs in multiple therapeutic fields. An efficient and practical high-throughput druggability development workflow implemented during antibody production and early screening is critical for selecting the best candidate molecules.
Traditionally, from early discovery to the development stage, there are only limited criteria (such as antigen binding and in vivo characteristics of animal models, including safety, pharmacokinetics, and pharmacodynamics) to select candidate mAbs.
If there is no extensive characterization to understand the biochemical and biophysical properties of the selected candidate, unexpected modification and stability in the later period may cause the project to delay or even terminate. Therefore, it is very important to evaluate the developability of the drug before entering the process development.
Comprehensive development evaluation focuses on the early stages of biochemical and biophysical properties evaluation, such as structure, stability, charge distribution, post-translational modification (PTM), solubility, and hydrophobicity.
In the production process of mAb, different physical and chemical factors are prone to produce various PTM variants, such as glycosylation, oxidation, deamidation, isomerization and so on. These PTMs may cause changes in the physical and chemical properties of antibodies, negatively affecting mAb potency, immunogenicity, and stability. Therefore, the PTM of mAb is closely related to the safety, effectiveness, and stability of the product.
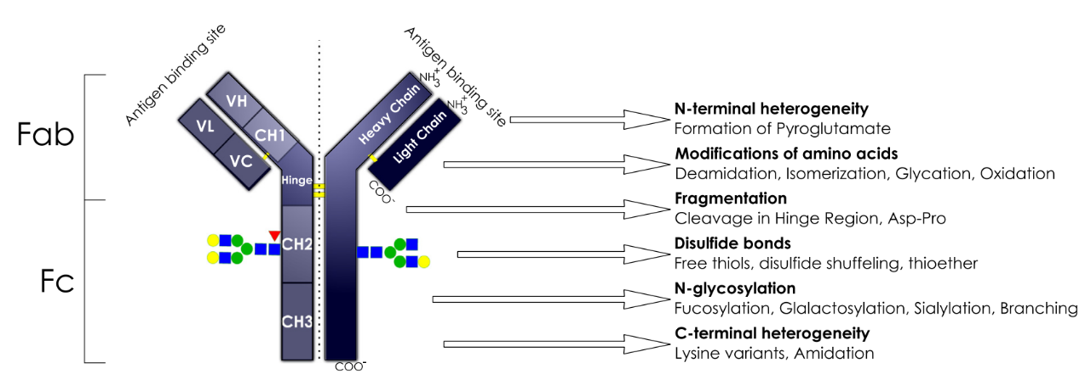
Deamidation
Asn deamidation is one of the most common degradation pathways in mAbs. Many drugs may undergo non-enzyme-catalyzed deamidation reactions during the production and storage processes, which will affect their structure and stability, resulting in a reduction or complete loss of the activity of the drug.
Isomerization
ASP isomerization is another common degradation pathway of mAb. Asp isomerization may also lead to a decrease in antigen-binding affinity.
Oxidation
Methionine (Met) and tryptophan (Trp) residues often undergo oxidation. The oxidation of CDR2 in the heavy chain and Met in the framework region does not affect antigen binding. However, higher oxidation levels or locations that are more critical for antigen binding may have a negative impact. The oxidation of Trp residues can lead to a decrease in potency and thermal stability, and an increase in aggregation tendency.
Glycosylation
Among the many PTMs, glycosylation is one of the most important and complex modifications, and also one of the key quality attributes when evaluating antibodies. In addition to the conservative N-glycosylation site (Asn-297) in the Fc region of mAb, it is not uncommon for N-glycosylation sequences (NXS/T, X cannot be P) to appear in the variable region. Variable region glycosylation has different effects on antigen binding but does not affect the half-life in vivo. Variable region glycosylation increases the uncertainty of the consistency of antibody efficacy and later development.
Creative Proteomics can provide you with the post-translational modification analysis to ensure the safety, effectiveness, and stability of your therapeutic proteins.






