The identification of protein modification sites and the identification of heterogeneity are the key links in the quality control of modified proteins, especially the analysis of PEGylation sites and identification of PEGylation heterogeneity are related to the pharmacokinetics, pharmacodynamic properties and stability of protein drugs. Therefore, the analysis of PEGylation sites and identification of PEGylation heterogeneity are important biochemical technologies in the research and development of polyethylene glycol modified protein drugs. MALDI-TOF MS and ESI-MS technology methods can be used to analyze of PEGylation sites and identify the PEGylation heterogeneity of drug proteins.
Creative Proteomics is a biopharmaceutical research partner with a first-class experimental equipment platform and rich biotechnology resources. We adopt mature proteomics research techniques and methods to provide you with various types of services. The structural characterization analysis and heterogeneity identification of protein drugs in biotechnology drugs are the key links in the quality control of protein drugs. In particular, the ICH Q6B guideline requires information on the protein structure and sequence of biological drugs. We will provide you with comprehensive and GLP / cGMP-compliant the analysis of PEGylation sites and identification of PEGylation heterogeneity service around the ICH guidelines (especially ICH Q6B) and the US FDA issues 'Points to Consider' document.
We Can Provide but Not Limited to:
- Analysis of the proportion of PEGylation sites
- Analysis of PEGylation degree in protein structure
- Identification of PEGylation heterogeneity
- Peptide sequence analysis
- Molecular mass analysis of protein
Technology Platform of PEGylation Sites and PEGylation Heterogeneity Identification Service:
Creative Proteomics provides accurate analysis of PEGylation sites and identification of PEGylation heterogeneity by providing matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS), electrospray ionization mass spectrometry (ESI-MS) technology, size exclusion chromatography, and isoelectric focusing electrophoresis.
We provide the following analysis methods:
➢ Analysis of PEGylation sites
1) MALDI-TOF MS analysis
Use MALDI-TOF MS technology to analyze the complete molecular weight and peptide sequence analysis of proteins in biopharmaceuticals to obtain the characteristic information of PEGylation sites and quantify the degree of modification of PEGylation.
2) ESI-MS analysis
Use ESI-MS technology to analyze the molecular mass of polyethylene glycol modified protein, and use the oligonucleotide sequence information in the target peptide to perform accurate quality assessment to analyze the average of the PEGylation sites. The specific workflow is as follows:
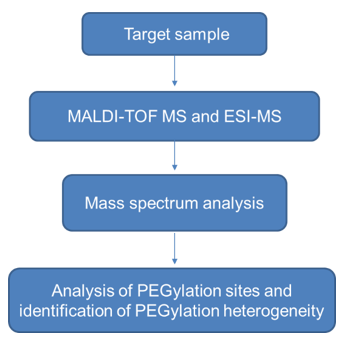
➢ Identification of PEGylation heterogeneity
Use protein purification technology to purify PGE-conjugated biomolecules, digest these proteins, and perform PEGylation degree of polyethylene glycol modification sites on the obtained target protein fragments, and finally calculate the potential positional isomers in PEGylated biomolecules to identity the heterogeneity of PEGylation. The specific workflow is as follows.
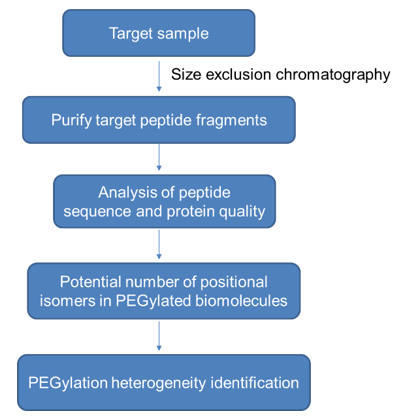
Advantages of PEGylation Sites and PEGylation Heterogeneity Identification Service:
- Short time-consuming: This technology uses a first-class experimental platform and simplifies sample preparation and experimental operation steps, which greatly reduces the identification and analysis time of the characteristic information of PEGylation sites.
- High sensitivity: MALDI-TOF MS and ESI-MS technologies have lower requirements for mass resolution and higher sensitivity.
- High accuracy: Mass spectrometry uses fragment mass spectrometry to generate smaller and more uniform peptide fragments in order to obtain accurate peptide sequence and mass analysis information.
- High-throughput: The combination of digestive protease in this service can ensure 100% coverage of any digested protein, and at the same time, it can identify thousands of different biological protein PEGylation sites and quantify the degree of PEGylation.
- Rapid turnaround time: 5-7 days to provide detailed technical reports.
- Customized service: We can customize professional solutions for you according to your research plan needs. You can select or suggest the required items for analysis.
Creative Proteomics's professional researchers can provide customers with comprehensive information on PEGylation sites in biopharmaceuticals and quantify the degree of PEGylation to achieve stable progress in the analysis of physical and chemical properties of biopharmaceuticals and the analysis of complete protein structures. We will provide you with a detailed experimental design plan and the final analysis report of PEGylation sites and polyethylene glycol heterogeneity and other bioinformatics analysis reports. We are committed to providing you with professional services on polyethylene glycol modified proteins in biotechnology drugs to help you solve problems related to analytical technology.
References
- Yoo C, Suckau D, et al. Toward top-down determination of PEGylation site using MALDI in-source decay MS analysis. J Am Soc Mass Spectrom. 2009.
- Lu X, Gough PC, et al. Elucidation of PEGylation site with a combined approach of in-source fragmentation and CID MS/MS. J Am Soc Mass Spectrom. 2010.






