Protein molecules often contain benzene ring structures such as tyrosine, tryptophan, and phenylalanine, and have a maximum absorption peak at the ultraviolet wavelength of 280nm. The light absorption value is directly proportional to the protein concentration, and the protein content can be determined by the absorption value at 280 nm wavelength.
For a certain recombinant protein drug, its maximum absorption wavelength is fixed. Generally, the ultraviolet absorption peak of protein drugs such as antibodies can be observed at about 280nm. And in the production process, the UV absorption spectrum of each batch of products should be consistent. Purity determination is also an important detection index for recombinant protein drugs, which directly reflects the level of antibody purification technology and product quality. To avoid the deviation of a detection method during protein purity detection, at least two separate methods should be used.
Bicinchoninic acid (BCA) method
BCA is a protein quantification method widely used in recent years. Its principle is that in an alkaline environment, protein is complexed with divalent copper ions and reduces the divalent copper ions to monovalent copper ions. BCA combines with monovalent copper ions to form a stable blue-violet complex with a higher absorbance value at 562 nm, which is proportional to the protein concentration.
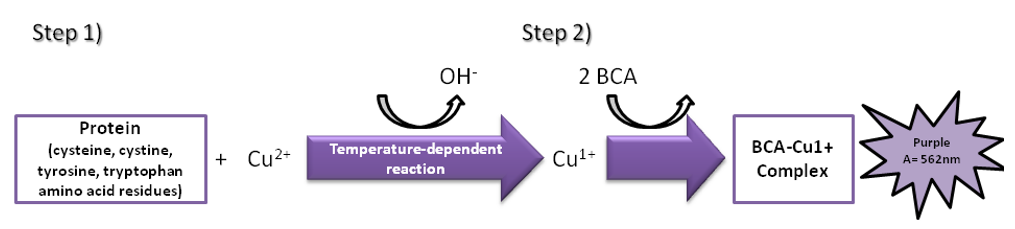
The BCA is highly sensitive for protein determination. It is simple to use and the color complexes formed with the reagents are very stable. The BCA method is suitable for the detection of protein concentration in the presence of surfactants and is compatible with up to 5% of SDS, TritonX-100 and Tween. However, since the BCA method relies on copper ions for the color reaction, if the solution contains chelating agents that react with copper ions such as EDTA or reducing agents such as DTT and β-mercaptoethanol, the results will be greatly affected. At the same time, the detection result can also be affected by the content of cysteine, tyrosine, and tryptophan in the protein.
UV spectrophotometry
Principle of UV spectrophotometry: The protein content is estimated by measuring the absorbance at 280nm of tyrosine and tryptophan containing conjugated double bonds in the protein. The protein concentration is calculated according to the Beer-Lambert law.
A=E•C•l.
A: absorbance, E: extinction coefficient, C: protein concentration, l: optical path length.
The extinction coefficient is the value of light absorption of the tested solution. If the concentration of the tested solution is high, the color is dark after the solution develops, the light absorption is large, the light transmittance is low, and vice versa. The same solution has different absorption peaks for spectra of different wavelengths. In order to improve sensitivity, the complementary color of light is generally selected as the preferred wavelength. For example, blue and yellow light are complementary colors. The 595nm wavelength is in this range, and the maximum absorption value can be measured, which improves the sensitivity. While 465nm is the wavelength of the green light,the blue solution has low absorption of green light and the sensitivity is naturally low. The extinction coefficient can be estimated from the protein sequence.
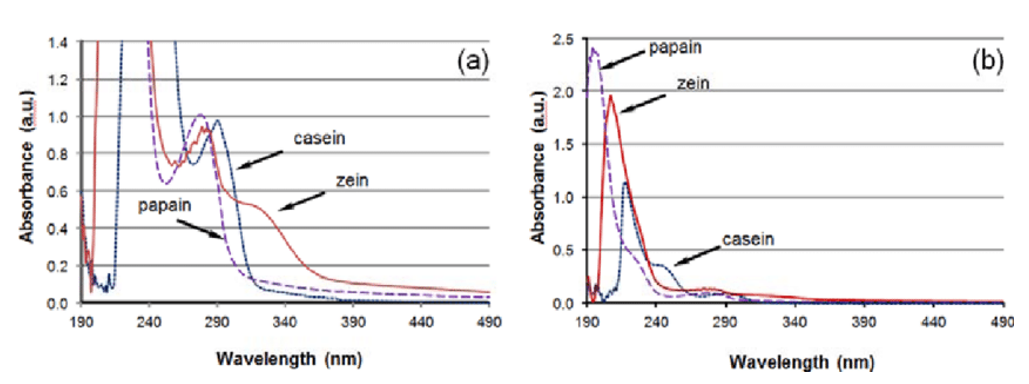
Some factors need to be considered when using UV spectrophotometry. First, different proteins contain different levels of tyrosine and tryptophan. Therefore, for accurate measurement, the pure product of the protein to be tested must be used as a reference. In addition, this method will be affected by substances with absorbance at 280 nm, including some buffer ions, nucleic acids, ethanol, and so on. Among them, the nucleic acid has the most serious impact. The maximum absorption peak of nucleic acid is at 260 nm. Therefore, when the nucleic acid is present in the solution at the same time, OD260 and OD280 must be measured at the same time, and then the ratio of the absorbance of the two wavelengths is corrected by an empirical formula to eliminate the influence of nucleic acid. This method is simple and rapid to operate, does not consume samples, and is mostly used for the trace determination of purified protein.






