Protein structure is critical to its function because it determines whether the protein can interact with other molecules. Since it is very complex, early structural biochemists conceptually divided protein structure into four "levels" to make it easier to grasp the complexity of the overall structure.
Protein primary structure
The primary structure of a protein is a unique sequence of amino acids in each polypeptide chain that constitutes the protein. This is just a list of the amino acid sequence in a polypeptide chain, not a structure. But since the final protein structure ultimately depends on this sequence, it is named the primary structure of the polypeptide chain.
The DNA sequence ultimately determines the unique sequence of amino acids in each peptide chain. Changes in the nucleotide sequence of a gene's coding region may cause a different amino acid to be added to the growing polypeptide chain, resulting in changes in the structure and function of the protein.
Protein secondary structure
The secondary structure of a protein refers to any regular structure produced by the interaction between adjacent amino acids when the polypeptide begins to fold into a functional three-dimensional form. The secondary structure appears in a region of the polypeptide chain to form hydrogen bonds between the partial groups of amino acids. There is rarely a secondary structure that extends to the entire polypeptide chain, usually only part of the chain. The most common forms are α-helix and β-sheet structures, which play an important structural role in most globular and fibrous proteins.
α-helix and β-sheet sheets form due to the hydrogen bond between the carbonyl group and the amino group on the peptide backbone. Certain amino acids tend to form α-helices, while other amino acids have a tendency to form β-sheets.
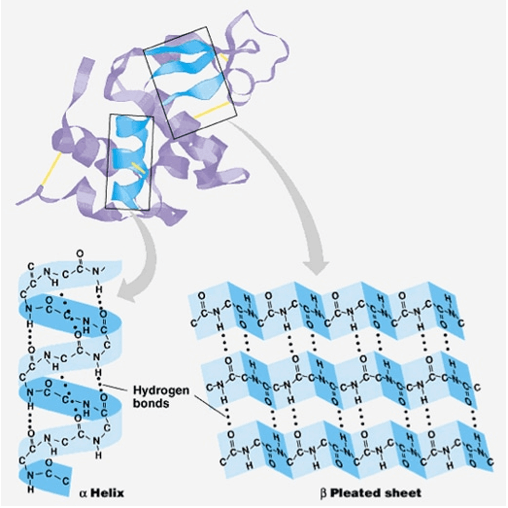
In an α-helical chain, hydrogen bonds are formed between the oxygen atom in the carbonyl group of the polypeptide backbone of one amino acid and the hydrogen atom in the amino group of the polypeptide backbone of another amino acid, the latter being the four further amino acids along the chain. This keeps the stretch of amino acids in the right-hand disk. Each spiral loop in the alpha helix has 3.6 amino acid residues. The R group (side chain) of the polypeptide extends from the α-helical chain and does not participate in the hydrogen bonding that maintains the α-helical structure.
In the β-folded sheet, due to the non-linear nature of single C-C and C-N covalent bonds, the extended part of amino acids is almost completely extended conformational "wrinkled" shape. β-pleated sheets never appear alone. They must be secured with other β-folding sheets. The amino acid fragment in the β-sheet remains in its sheet structure by hydrogen bonds formed between the oxygen atom in the carbonyl group of the peptide backbone of one β-sheet and the hydrogen atom in the amino group of the peptide backbone of the other β-sheet. The sheets of β-folds are connected and arranged parallel or antiparallel to each other. The R groups of the amino acids in the β-sheet point perpendicular to the hydrogen bonds holding the β-sheet together and are independent of maintaining the β-sheet structure.
Protein tertiary structure
The tertiary structure of a polypeptide chain refers to the overall three-dimensional shape of all the secondary structure elements folded with each other. The interaction between the polar, non-polar, acidic and basic R groups in the polypeptide chain forms a complex three-dimensional tertiary structure. When a protein is folded in an aqueous environment in the body, the hydrophobic R groups of non-polar amino acids are mainly located inside the protein, while the hydrophilic R groups are mainly located outside the protein. Cysteine side chains form disulfide bonds in the presence of oxygen, which are the only covalent bonds formed during protein folding. All these interactions, whether weak or strong, determine the final three-dimensional shape of the protein. A protein can no longer function if it loses its three-dimensional shape.
Protein quaternary structure
The quaternary structure of a protein refers to how its subunits are oriented and arranged with each other. Therefore, the quaternary structure is only suitable for multi-subunit proteins, that is, proteins composed of more than one polypeptide chain. A protein synthesized from a single peptide will not have a quaternary structure.
In proteins containing multiple subunits, the weak interaction between the subunits helps stabilize the overall structure. Subunits are usually the key enzymes that form the final function.
Creative Proteomics can provide you with protein sequence analysis, secondary structure analysis, and tertiary structure analysis services. Our analytical scientists can provide results quickly, succinct written reports, and customized services to help our customers achieve the perfect solution.






