The 1H (proton) NMR spectra are one of the most common NMR experiments. Apart from the tritium, the proton is the most sensitive nucleus and usually yields sharp signals. Proton NMR is very useful depending on its sharp signals even though its chemical shift range is narrow. 1H NHR determines the structure of molecules by used proton. The 1H NMR spectra usually provide the following information:
- Number of signals
A separate signal in the NMR spectrum can be obtained as a result of protons within different compound experiencing different magnetic environments.
- Position of signals (chemical shift)
Protons usually sense three different magnetic field including Earth magnetic field, the NMR spectra and the magnetic field from neighboring protons. However the chemical shift depends only on the varying magnetic fields of protons in molecules because of similar effect of the other two magnetic fields.
- Relative intensity of signals (integration)
A signals area is related to the number of protons and thus the relative intensity of signals represents the relative number of proton equivalents.
- Splitting of signals
The interaction between neighboring protons produce different spin flip energies and splitting may offer information on the nearby proton with the target analyte.
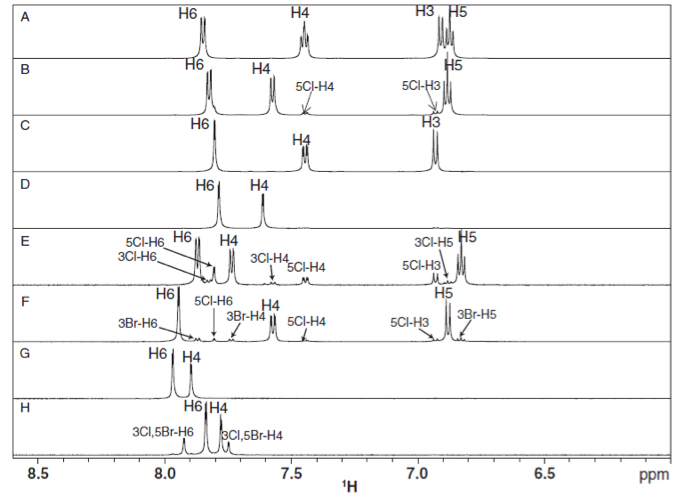
Figure 1H NHR spectra of halogenated salicylic acid compounds[1]
[1] 1H and 13C NMR spectral assignments of halogenated transformation products of pharmaceuticals and related environmental contaminants. Solakyildirim K, Magn Reson Chem., 2014, 52(6).






