Circular dichroism (CD) is a powerful analytical technique that can characterize secondary structural elements in peptides, such as α-helices, β-sheets, and random coils. With our expertise and cutting-edge equipment, Creative Proteomics provides precise and reliable analysis to help researchers and pharmaceutical companies reveal the structural properties of peptides for drug discovery, protein engineering and biophysical research. Trust us to unlock the secrets of peptide structure and guide your scientific endeavors to success with our advanced CD spectroscopy services.
Our CD Technology Platform
Circular dichroism (CD) is the most widely used method for determining the secondary structure of peptides. Different parts of the peptide chain can form specific structures such as α-helices, β-sheets, and random coils. At ultraviolet 185 ~ 240 nm, the elliptical value wavelength variation curves of several different structures are different. As shown in the figure below, the circular dichroism spectrum in this wavelength range can be used to study the content of various secondary structures in peptides.
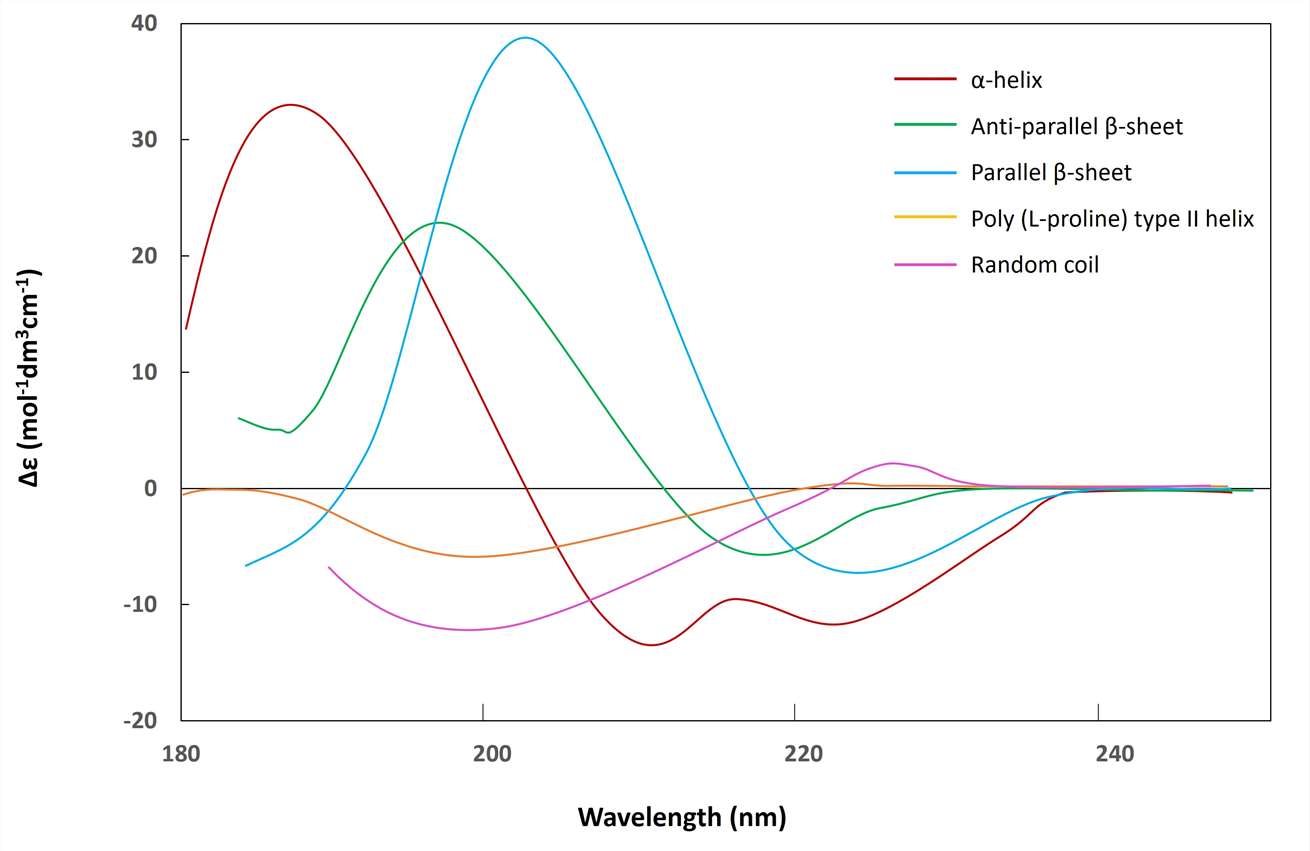 Circular Dichroism Chromatograms - Secondary Structures of Proteins/Peptides (Rodger, A.; et al. 2005)
Circular Dichroism Chromatograms - Secondary Structures of Proteins/Peptides (Rodger, A.; et al. 2005)
Advantages of CD Technology
- It can be measured in solution state, which is closer to its physiological state.
- Quick and easy assay.
- Sensitive to conformational changes.
Process of Our Peptide Secondary Structure Analysis
Prepare Peptide Samples
The peptide concentration in the sample is generally 0.3 ~ 0.5 mg/mL, and the buffer in which the peptide is located is generally phosphate buffer.
Circular Dichroism Preheating
After the circular dichroism spectrometer is turned on, it needs to be filled with nitrogen for about 30 minutes, and then the hernia lamp is turned on. Set the scanning wavelength range: 180 nm ~ 260 nm, repeat 2 times.
Detecting Instrument Stability
Directly scan the air to obtain a straight line with a range around 0.1, which is considered normal, and then detect the corresponding buffer curves respectively.
Detection of Sample Secondary Structure Folding
Pipette 200 μL of sample into a cuvette with a thickness of 1 mm. Observe through light to avoid bubbles. The temperature is 20°C. The curve can be obtained after scanning.
Detection of Peptide Thermal Stability (Tm)
The cold cycle controller needs to be turned on. Pipette 200 μL protein sample into a cuvette with a thickness of 1 mm. Observe through light to avoid air bubbles. Insert the temperature probe into the sample. The temperature range is 20°C ~ 95°C, and detection is performed every 5°C. Each test was repeated 2 times. According to the initial secondary structure spectral curve of the protein, take the data at 220 nm or 222 nm and use GraphPad Prism 5.0 to make a graph, fit and calculate Tm.
Our Sample Requirements for CD Experiments
- The sample needs to ensure a certain degree of purity, it is required to contain no light-absorbing impurities, and the solvent must have no absorption interference at the measurement wavelength.
- The sample can be completely dissolved in solvent to form a uniform and transparent solution.
- The sample concentration is generally 0.05 ~ 0.5 mg/mL. Too high a concentration and too much noise will affect the results.
- Different samples have different measured circular dichroism spectral ranges, and the requirements for the selection of pool size (optical path) and concentration are also different.
Are you seeking an accurate and comprehensive understanding of peptide structure? Look no further than Creative Proteomics, where we specialize in peptide structure analysis using circular dichroism (CD) spectroscopy. Contact us today to discover how our expert CD spectroscopy services can support your scientific career and unlock the mysteries of peptide structure.
Reference
- Rodger, A.; et al. Circular dichroism spectroscopy for the study of protein-ligand interactions. Methods Mol Biol. 2005, 305:343-64.
* For research use only. Not for use in diagnostic procedures!
Our customer service representatives are available 24 hours a day, 7 days a week.

 Circular Dichroism Chromatograms - Secondary Structures of Proteins/Peptides (Rodger, A.; et al. 2005)
Circular Dichroism Chromatograms - Secondary Structures of Proteins/Peptides (Rodger, A.; et al. 2005)