Product-Related Impurity Analysis
Online Inquiry
Product-related impurity analysis and characterization is an important aspect of process validation for antibody drug development. It identifies and characterizes molecular variants that differ from the desired drug in terms of activity, efficacy and safety. Creative Proteomics uses a variety of advanced techniques in accordance with the ICH Q6B guidelines to help customers fully understand these product-related impurities in order to assess their impact on product efficacy and safety.
Effects of Product-Related Impurity of Antibody Drugs
The generation of relevant substances or impurities in antibody therapeutic products can affect the affinity purification process, the distribution and metabolic characteristics of the products in tissues and sera, the binding ability of the Fab and Fc segments of the antibodies to the corresponding antigens and receptors respectively, as well as the immunogenicity of the products. Therefore, the study of related substances and impurities is an important element to ensure the safety, efficacy and quality controllability of the products and should be emphasized.
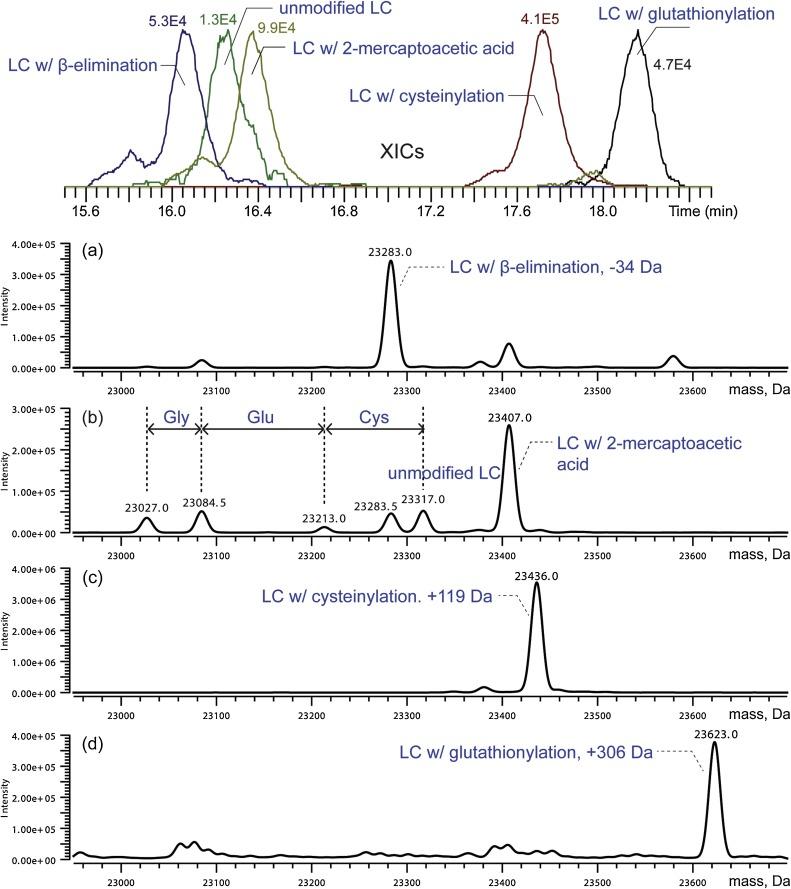 Fig. 1 Light chain variants identified in the HILIC-MS analysis of mAb-1 sample. (Wang, S. et al., 2018)
Fig. 1 Light chain variants identified in the HILIC-MS analysis of mAb-1 sample. (Wang, S. et al., 2018)
What We Test?
Antibody drug products can contain various impurities, which could arise from various sources, during the manufacturing process, storage, or degradation, including, among others, including aggregates, monomeric variants, post-translational modifications (PTMs) such as glycosylation, oxidation, deamidation, and protein hydrolysis fragments. Our company specializes in providing comprehensive impurity analysis and characterization services for antibody drug products.
Aggregate Analysis
The presence of aggregated or misfolded antibody molecules can negatively impact the drug's efficiency and immunogenicity, potentially reducing its therapeutic effectiveness. We can isolate aggregates from the product to be tested and quantify them by size exclusion chromatography or capillary electrophoresis.
Post-Translational Modification Analysis
Post-translational modification processes (e.g., glycosylation modifications, deamidation, oxidation)) often produce antibody variants that may be product-related impurities that affect antibody stability, potency, half-life, and immunogenicity. Our experienced analytical team performs detailed analysis and characterization of impurities resulting from post-translational modifications using a variety of techniques including chromatography, isoelectric focusing (IEF), peptide mapping, MS-MS and NMR.
Fragment Variants Analysis
Antibodies might undergo fragmentation, leading to the generation of fragments or species with modified biological activities, affecting the desired pharmacological response. Fragment products are produced during secretion, expression, purification and preservation by conditions such as primary sequence, hydrophobicity, chargeability, shear in the manufacturing process, formulation prescription, protein concentration, pH, temperature, vibration, and repeated freezing and thawing. For these fragment variants, we enzymatically or chemically cleave the peptide and then characterize it by chromatography or SDS-PAGE. In addition, we can combine this with peptide map analysis to generate useful information about the variants.
Advantages of Our Product-Related Impurity Analysis Services
- Expertise in antibody drug products
- State-of-the-art equipment
- Comprehensive impurity profiling
- Regulatory compliance
- Fast turnaround time
Creative Proteomics understands the importance of identifying, characterizing and quantifying impurities to ensure the safety, efficacy and quality of antibody drugs. We are committed to providing our customers with comprehensive impurity analysis and characterization services. For more information or to request our services, please feel free to contact us.
Reference
- Wang, S.; et al. Characterization of product-related low molecular weight impurities in therapeutic monoclonal antibodies using hydrophilic interaction chromatography coupled with mass spectrometry. Journal of pharmaceutical and biomedical analysis. 2018, 154: 468-475.

 Fig. 1 Light chain variants identified in the HILIC-MS analysis of mAb-1 sample. (Wang, S. et al., 2018)
Fig. 1 Light chain variants identified in the HILIC-MS analysis of mAb-1 sample. (Wang, S. et al., 2018)