Host Cell Protein Residue Analysis
Online Inquiry
Host cell protein residue (HCP) analysis is required for quality control and safety measures in the development of antibody drugs. Creative Proteomics provides efficient and professional HCP detection and analysis services in accordance with the requirements of the U.S. Pharmacopeia (USP) and other International Council for Harmonization (ICH) guidance documents, including Q6B, on strategies for monitoring HCP impurities. Our testing platform is equipped with advanced analytical equipment and methods to provide comprehensive HCP assay services to support antibody drug development programs at any stage.
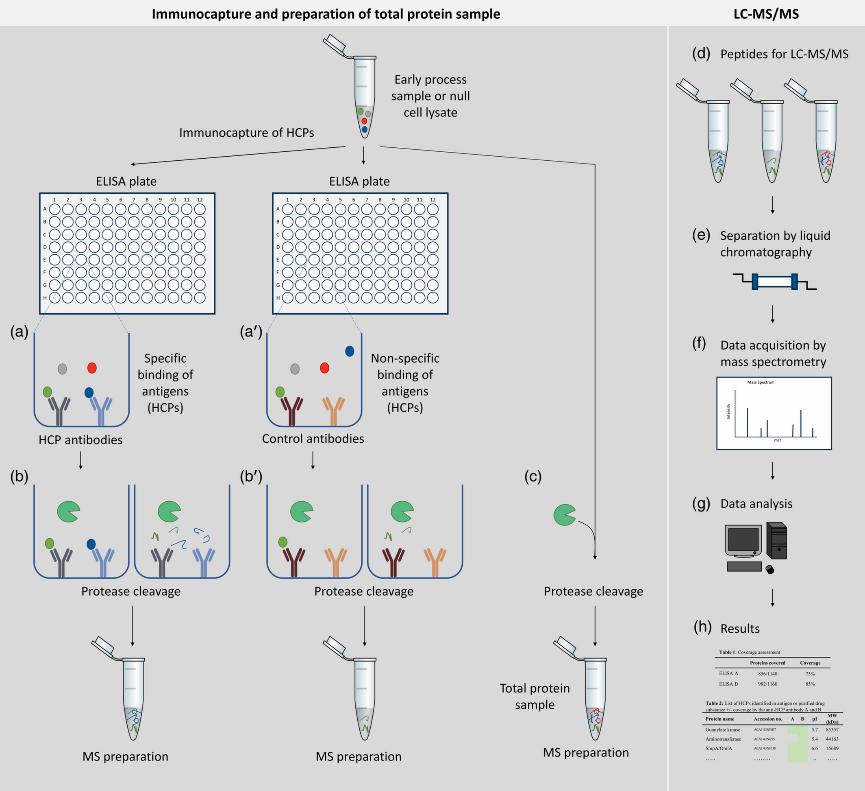 Fig. 1 A novel approach to evaluate ELISA antibody coverage of host cell proteins. (Pilely, K. et al., 2020)
Fig. 1 A novel approach to evaluate ELISA antibody coverage of host cell proteins. (Pilely, K. et al., 2020)
Importance of HCP Testing in Antibody Drugs
Host cell proteins (HCPs) are a major class of process-related impurities and require monitoring and documentation of their presence through antibody drugs development and manufacturing. While such residual proteins are typically eliminated during the purification process, trace levels can still remain. Even in residual amounts, they are known to affect product quality and efficacy as well as patient safety. These contaminants can elicit an unwanted immune response or alter the efficacy and safety of the drug, thus necessitating the need for their detection and quantification.
Our Host Cell Protein Residue Analysis Services
- Process-specific HCP development services
Setting up a strategy for HCP analysis is a complex undertaking, as HCPs are a complex group of impurities composed of thousands of different proteins present at cell harvest that may co-purify with the drug through downstream processing. Creative Proteomics has extensive experience in HCP assays for biopharmaceuticals, and we are able to provide customized assays based on the stage of the project and the needs of different clients. When the manufacturing process is scaled up and the purification process is changed during development, the HCP assays we offer are able to measure any new HCP without the need to develop a new assay.
- Development and validation of antibody drug process-related HCP assays.
We complete analytical method validation/validation and support analytical method transfer in accordance with ICH, Food and Drug Administration and other relevant guidelines.
- Coverage assessment for upstream proprietary process HCP.
ELISA's ability to cover the HCPs in the process must be evaluated by an HCP coverage analysis. We used 2D-PAGE and Western blotting methods to evaluate the coverage of HCP antibodies against HCP in process proprietary HCP detection kits.
- Characterization of HCP samples by mass spectrometry.
For intermediate processes and antibody drug stock samples, we offer mass spectrometry detection as an orthogonal method for HCP identification and content detection.
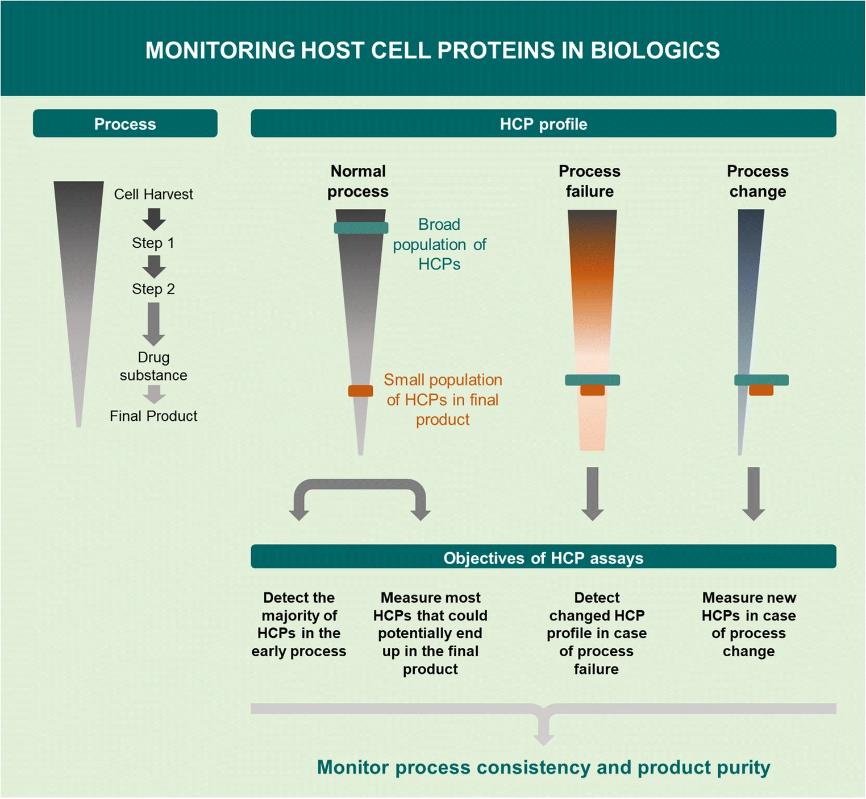 Fig. 2 Objectives of HCP assays for monitoring HCPs in biologics. (Pilely, N. et al., 2022)
Fig. 2 Objectives of HCP assays for monitoring HCPs in biologics. (Pilely, N. et al., 2022)
Our Technologies for HCP detection and identification
Since HCPs are a highly diverse group of impurities, several technologies and regulatory requirements for HCP analysis exist. Our platform has established a variety of highly sensitive assays. The methodology employed for Host Cell Residue Protein Assay Analysis incorporates the use of enzyme-linked immunosorbent assay (ELISA), the gold standard for HCP testing. It is highly sensitive and capable of detecting HCPs at very low levels. We also utilize mass spectrometry, amino acid analysis and other protein chemistry techniques to identify and quantify HCPs, should a more in-depth analysis be required.
Our Technological Advantages
- High sensitivity.
- Wide dynamic range.
- Easy to perform, reproducible quantification.
HCP Testing Steps
The testing steps mainly involve the extraction of proteins, followed by detection and quantification steps. Initially, the proteins are extracted from the final drug product, purified and then analyzed through ELISA or other protein chemistry methods mentioned earlier. The detection and quantification of HCPs involve the use of specific antibodies to these proteins, and the degree of antigen-antibody reaction is measured.
Creative Proteomics provides host cell residue detection and analysis services to meet the demands of antibody drug manufacturers and R&D companies to ensure drug compliance, safety and efficacy. For more information or to request our services, please feel free to contact us.
References
- Pilely, K.; et al. Monitoring process-related impurities in biologics-host cell protein analysis. Analytical and Bioanalytical Chemistry. 2022, 414(2): 747-758.
- Pythoud, N.; et al. Optimized sample preparation and data processing of data-independent acquisition methods for the robust quantification of trace-level host cell protein impurities in antibody drug products. Journal of Proteome Research. 2020, 20(1): 923-931.
- Pilely, K.; et al. A novel approach to evaluate ELISA antibody coverage of host cell proteins-combining ELISA‐based immunocapture and mass spectrometry. Biotechnology progress. 2020, 36(4): e2983.

 Fig. 1 A novel approach to evaluate ELISA antibody coverage of host cell proteins. (Pilely, K. et al., 2020)
Fig. 1 A novel approach to evaluate ELISA antibody coverage of host cell proteins. (Pilely, K. et al., 2020) Fig. 2 Objectives of HCP assays for monitoring HCPs in biologics. (Pilely, N. et al., 2022)
Fig. 2 Objectives of HCP assays for monitoring HCPs in biologics. (Pilely, N. et al., 2022)