Dynamic Light Scattering (DLS)
Online Inquiry
In the process of antibody drug characterization, the determination of protein particle size is important for its stability assessment, protein interaction studies, and protein aggregation analysis. Dynamic light scattering (DLS) is a key characterization technique for biopharmaceutical, providing information including particle size, shape and diffusion. Creative Proteomics offers comprehensive solutions for antibody drug characterization with our advanced DLS technology. Our systems are equipped with advanced detectors and data analysis systems with high sensitivity. With the advantages of short assay time and low sample preparation requirements, we help our customers save time and resources and accelerate the process of antibody drug development.
Principle of DLS
DSL is one of the most commonly used analytical methods for determining particle size and radius distributions in suspensions or polymers in solution samples. In the context of DLS, temporal fluctuations are usually analyzed by intensity or photon autocorrelation function (ACF). A monochromatic beam of light (e.g., a laser) irradiated into a test solution containing spherical particles moving in Brownian motion induces a Doppler shift when the light strikes the moving particles, thereby changing the wavelength of the original light. This change, is related to the size of the particle. By measuring the diffusion coefficient of the particles in the test medium by ACF, the size distribution of the spheres can be calculated and the motion of the particles in the test medium can be described in detail.
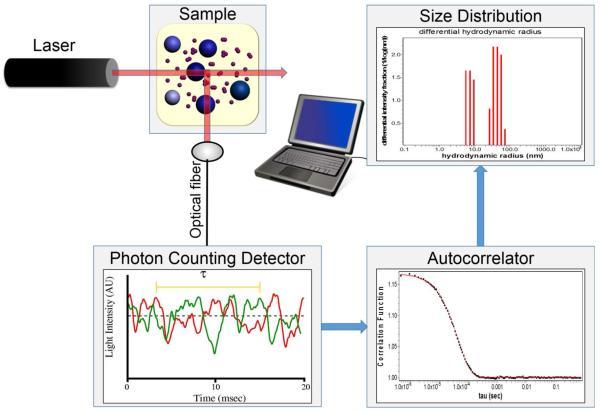 Fig. 1 Schematic of a system for measurement and analysis of dynamic light scattering. (Minton, A. P., 2016)
Fig. 1 Schematic of a system for measurement and analysis of dynamic light scattering. (Minton, A. P., 2016)
Applications of DSL in Antibody Drug Characterization
Aggregation analysis
DLS can rapidly measure the size distribution and quantify the extent of aggregation in a sample. By determining the size and polydispersity index (PDI), we can determine the presence of aggregates, oligomers, or other large particles that may affect the quality of the drug.
Stability assessment
DLS monitors changes in antibody particle size and distribution over time to reveal its stability profile. Our characterization experts provide insight into the stability of pharmaceutical formulations by quantifying increases in particle size or aggregation, optimizing storage conditions and shelf life.
Conformational changes analysis
By measuring the hydrodynamic radius, DLS can provide information about the conformation of the antibody molecule. We can analyze conformational changes to understand unfolding, denaturation, or the formation of new conformations, and even detect subtle conformational changes that may affect an antibody's binding affinity or biological activity.
Advantages of Our DSL Analysis
- Accurate, reliable and reproducible particle size analysis.
- Simple sample preparation, even natural samples can be analyzed directly without sample preparation.
- Simple operation and fully automated measurement.
- Measures sizes smaller than 1 nm.
- Measures molecules with molecular weights <1000 Da.
- Low volume requirements.
Our Capabilities
- Quantitative analysis: perform quantitative analysis using advanced DLS techniques to assess the aggregation state and stability of antibody drugs by measuring hydrodynamic radius, polydispersity index, and particle concentration.
- Method development: provides sample preparation and optimization of measurement conditions for DLS experiments in antibody drug characterization, including protein concentration, buffer composition, temperature and acquisition time, and other factors that may affect DLS measurement results.
- Well analyzed reports: Expert data interpretation provides comprehensive, detailed characterization reports.
Creative Proteomics is committed to providing state-of-the-art DLS solutions for antibody drug characterization. Contact us to learn more about our service and we will be happy to serve you.
Reference
- Minton, A. P. Recent applications of light scattering measurement in the biological and biopharmaceutical sciences. Analytical biochemistry. 2016, 501: 4.

 Fig. 1 Schematic of a system for measurement and analysis of dynamic light scattering. (Minton, A. P., 2016)
Fig. 1 Schematic of a system for measurement and analysis of dynamic light scattering. (Minton, A. P., 2016)