C-Terminal Lys Deletion Analysis
Online Inquiry
An important aspect of antibody drug development is the analysis of specific modifications or mutations that can enhance or alter the function of an antibody. Among these, C-terminal Lys deletions can have a variety of effects on antibody drugs. Creative Proteomics specializes in providing advanced analytical services for the characterization of C-terminal modifications in antibody drugs. Our expertise and state-of-the-art equipment enable us to provide accurate and reliable results that help pharmaceutical companies meet regulatory requirements and ensure the safety and efficacy of their antibody therapeutics.
Importance of C-Terminal Lys Deletion Analysis
The C-terminal Lys residue of the human serum IgG H-chain is usually cleaved in vivo, exposing the preceding Gly residue. Cleavage of Lys is thought to be catalyzed by endogenous carboxypeptidases in secretion or circulation. Individual antibodies in a production lot may therefore vary in the presence or absence of Lys residues at their C-terminus. C-terminal Lys may be present on both heavy chains of the antibody, on one of the heavy chains, or on neither. Loss of C-terminal Lys results in a change in the check digraph of the heterogeneity of the reactive charge, such as CEX or CIEF, which can affect the pharmacokinetics and therapeutic efficacy of the antibody. kinetics and efficacy. Therefore, it remains an important aspect in the characterization of antibody drugs.
C-Terminal Lys Deletion Analysis Services at Creative Proteomics
Creative Proteomics has a comprehensive suite of technologies and expertise to provide different methods to detect and quantify C-terminal Lys deletions, providing valuable insights into the characterization and potential therapeutic applications of antibody drugs. Among them, mass spectrometry is our commonly used analytical technique to detect and quantify C-terminal modifications at the molecular level. Our experienced researchers can identify and characterize C-terminal Lys deletions by analyzing antibody samples.
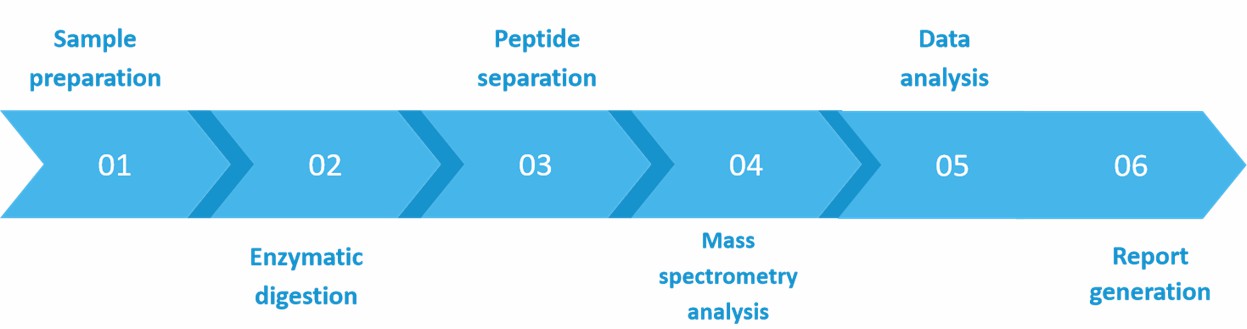
C-Terminal Lys Deletion Analysis Services Services Process
Our analysis service typically involves the following steps:
- Sample preparation
- Enzymatic digestion
Targeting the C-terminal region of the antibody and selecting the appropriate enzyme for enzymatic hydrolysis of the antibody drug sample allows for the release of the individual C-terminal Lys.
The digested sample is then separated using liquid chromatography (LC) techniques, typically employing reverse-phase chromatography.
- Mass spectrometry analysis
The isolated C-term Lys peptides are subjected to mass spectrometry (MS) analysis. MS detects and measures the mass-to-charge ratio of ions, providing valuable information about the peptide sequences and modifications.
Our advanced data analysis software allows for a comprehensive analysis of the acquired mass spectrometry data. Our experienced experts identify and quantify the C-terminal Lys peptides present in the antibody drug sample and assess any differences or changes in C-terminal Lys content.
In conjunction with the analytical results, we provide our clients with a full analytical report including information on the identified C-terminal Lys peptides, their abundance, and any observed modifications or changes.
Creative Proteomics provides our clients with authentic, reliable and timely characterization analysis with our advanced technology platform. Contact us to learn more about our C-terminal Lys deletion analysis services of antibody drugs. We will be happy to assist you.

