Antibody Drug Modification Analysis
Online Inquiry
Post-translational modifications are common testing program in the characterization of acid-base peaks of antibody drugs and in product comparability studies. The ICH-Q6B guideline requires that biopharmaceuticals should be analyzed for deamidation, phosphorylation, glycosylation, oxidation, and other forms of modification to develop acceptance quality criteria. Creative Proteomics offers a wide range of tools for characterizing post-translational modification types and content changes that accurately identify and control key attributes affecting the safety, stability and efficacy of antibody drugs to ensure compliance.
Why Characterize Post-Translational Modifications of Antibody Drugs?
Antibody molecules are subject to post-translational modifications during secretion, expression, purification and storage by the intracellular and extracellular environments, and common modifications include oxidation on methionine (Met), deamidation on asparagine (N), cyclization on the N-terminal Q (pyroQ), and loss of lysine at the C-terminal, etc. These modifications cause changes in the local conformation and charge of the antibody, which exhibit different ratios of acid-base peaks on the IEF. Modifications occurring in the CDR affect activity and cause a decrease in the therapeutic effect of the antibody drug. Therefore, a thorough understanding of the various post-translational modifications is important for preclinical and clinical development and commercial supply of antibody drugs.
Introduction of Post-Translational Modification Analysis Services
Antibody-based therapeutics can be modified in a variety of ways during production, purification. Creative Proteomics, through High Performance Liquid Chromatography (HPLC)-High Resolution Mass Spectrometry (HRMS) and advanced analytical software, can accurately characterize the sites and relative quantities of various modifications that occur, including, but not limited to, the following:
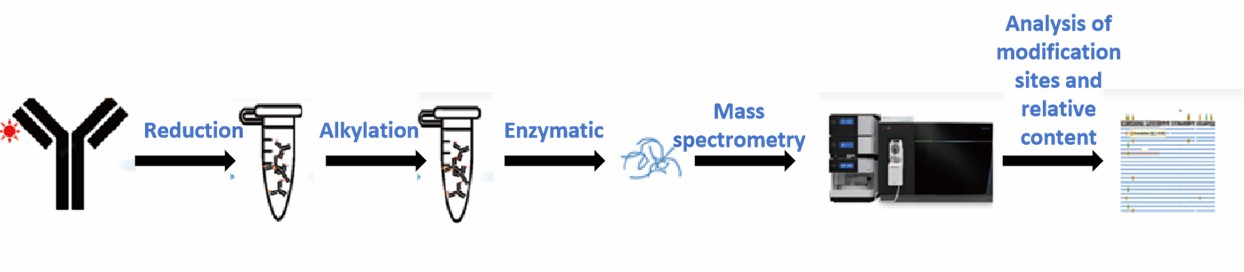
Technological Processes at Creative Proteomics
Creative Proteomics provides customized one-stop characterization solutions based on your needs, including the entire process of sample processing, mass spectrometry detection, and data analysis. The samples analyzed for post-translational modifications and sequence coverage were enzymatically digested in a similar manner. We calculate the sites and ratios of post-translational modifications based on sequence comparison results to provide accurate modification information.
Advantages of Our Post-Translational Modification Analysis Services
- The professional team is familiar with the key points of the analysis process, avoiding the interference and error brought by manual operation.
- The standardized operation process ensures fast, accurate service and delivery of results.
- High-resolution equipment and advanced data analysis system can dig deep into the post-translation modification information.
What We Deliver?
| Sample Requirements |
Deliverables |
Turnaround Time |
- Samples are freshly prepared and more than 90% pure.
- Samples are preserved in stable buffer and free of glycerol, SDS, etc.
- Sample volume not less than 100ug.
- Protein amino acid sequence.
|
Data analysis report: provide information on all detected plausible post-translational modification sites, relative abundance, etc., and give the 10 modification secondary profiles with the highest abundance and credibility. |
14 working days |
Creative Proteomics provides our clients with authentic, reliable and timely characterization analysis with our advanced technology platform. Contact us to learn more about our post-translational modification analysis services. We will be happy to assist you.

