Mass Spectrometry for Antibody Drug Characterization
Online Inquiry
Modern analytical technologies are rapidly advancing the characterization of biopharmaceuticals. Among them, the utility of mass spectrometry (MS)-based methods for antibody drug characterization is evolving. MS is recognized as one of the key technologies due to its unique analytical sensitivity, specificity, and selectivity. Creative Proteomics provides customers in the field of antibody drug development with state-of-the-art mass spectrometry tools for profiling the structural properties, post-translational modifications, purity and impurity profiles, and pharmacokinetic properties. Our experienced experts develop and apply mass spectrometry methods and strategies to help customers solve their antibody drug characterization challenges to accelerate the drug development process.

Application of Mass Spectrometry in Characterization of Antibody Drugs
Mass spectrometry has emerged as an important analytical tool in the development of therapeutic antibody drugs, allowing the identification, characterization, and quantification of a wide range of critical quality attributes, including structural integrity, stability, higher-order structure, and in vivo characterization. Our expert team utilizes mass spectrometry to assist in the discovery and development of antibody drugs, providing valuable information for in-process, release and stability purposes in the following analyses.
Primary Structure Characterization
One of the primary uses of mass spectrometry in antibody drug characterization is in the determination of the primary structure of antibodies. Mass spectrometry can identify the individual amino acids present in the protein sequence, allowing for accurate determination of the antibody's primary structure. This information is crucial for understanding the antibody's function and for quality control purposes.
Higher Order Structure Characterization
Higher order structure characterization is another application of mass spectrometry in antibody drug characterization. It can be used to probe the higher-order structures of antibodies, including the determination of disulfide bond connectivity, identification of covalent modifications, and assessment of conformational changes. By analyzing the mass spectral data, researchers can gain insights into the structural integrity of antibody drugs and detect any modifications or deviations from the expected structure. This information is valuable for understanding the stability and activity of the antibody.
Post-Translational Modification Characterization
Antibodies often undergo various PTMs, such as glycosylation, oxidation, and deamidation, which can influence their stability, efficacy, and immunogenicity. Mass spectrometry can accurately identify and quantify these modifications, providing valuable insights into their impact on antibody drug characteristics.
Determination of the Drug-to-Antibody Ratio (DAR) in ADCs
DAR is one of the most important properties of antibody drug conjugates. Mass spectrometry can precisely determine the number of drug molecules conjugated to each antibody, providing critical information on the drug loading efficiency and homogeneity of ADCs. This is crucial for ensuring the consistency and efficacy of ADC-based therapies.
Impurity Analysis
During the manufacturing process of antibody drugs, various impurities may be introduced, such as truncated or misfolded antibodies, incomplete drug conjugation, or degradation products. Mass spectrometry techniques, such as peptide mapping and intact mass analysis, can identify and quantify these impurities, which is essential for drug quality control and regulatory compliance.
Pharmacokinetic Studies
Understanding how the drug is distributed, metabolized, and eliminated from the body is crucial for optimizing dosing regimens and predicting efficacy. Mass spectrometry can quantify the concentrations of antibody drugs and their metabolites in biological samples, such as plasma or tissues, providing valuable information on drug clearance, half-life, and tissue distribution.
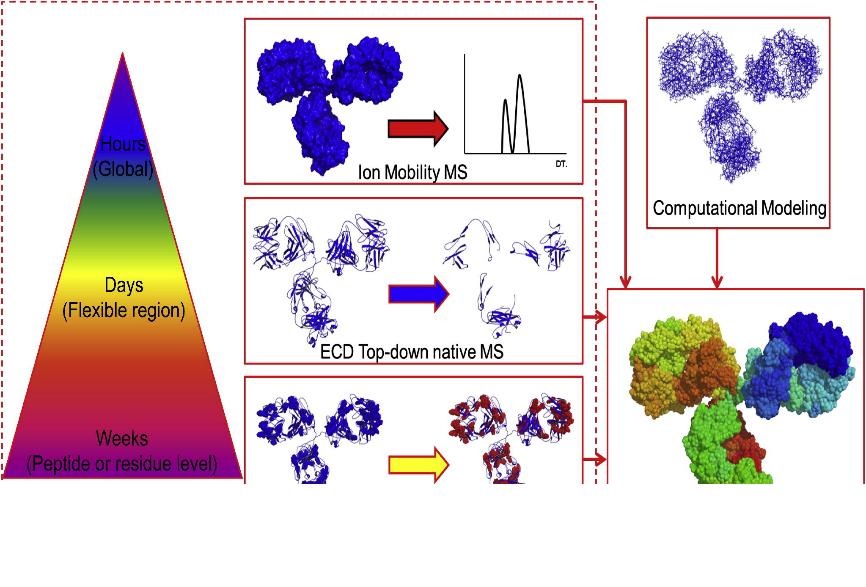 Fig. 1 The MS-based biophysical "tool box" in characterizations of IgG2 with different S-S bond networks. (Zhang, H. et al., 2014)
Fig. 1 The MS-based biophysical "tool box" in characterizations of IgG2 with different S-S bond networks. (Zhang, H. et al., 2014)
Advanced Mass Spectrometry for Antibody Drug Characterization
The application of mass spectrometry to characterize antibody drug composition and structure-related information is growing rapidly. With advances in instrumentation and analytical methods, mass spectrometry has become more sensitive and accurate and can be applied to characterize antibody drugs of various complex structures with high throughput. At Creative Proteomics, we offer a wide range of advanced mass spectrometry technologies and provide customized characterization strategies such as high-resolution mass spectrometry combined with top-down or bottom-up modes. We keep abreast of industry trends and new technologies, offering a variety of new analytical tools to characterize antibody drug products at every step of the process, from preparation to storage and shipping. Our advanced mass spectrometry technology provides a powerful approach to powering antibody drug development. Below are the advanced mass spectrometry tools we offer in the characterization of antibody drugs:
Advantages of Creative Proteomics
- Optimal sensitivity and resolution ensure high-quality data acquisition.
- Customized mass spectrometry workflows allow for high throughput analysis in a short period of time.
- Professional data interpretation and comprehensive analysis reports help clients make informed decisions early in drug development.
Creative Proteomics specializes in advanced mass spectrometry (MS) solutions for antibody drug characterization. Our expertise in this field ensures that our customers receive the most accurate and reliable data during drug development and quality control. We work closely with our customers to meet technical requirements and ensure rapid project delivery to support the rapid growth of the antibody drug industry. Contact us to learn more about our service and we will be happy to serve you.
References
- Zhang, H.; et al. Mass spectrometry for the biophysical characterization of therapeutic monoclonal antibodies. FEBS letters. 2014, 588(2): 308-317.
- Sandra, K.; et al. Modern chromatographic and mass spectrometric techniques for protein biopharmaceutical characterization. Journal of chromatography A. 2014, 1335: 81-103.


 Fig. 1 The MS-based biophysical "tool box" in characterizations of IgG2 with different S-S bond networks. (Zhang, H. et al., 2014)
Fig. 1 The MS-based biophysical "tool box" in characterizations of IgG2 with different S-S bond networks. (Zhang, H. et al., 2014)