Unconjugated Antibody Determination
Online Inquiry
The level of unconjugated antibody in an ADC formulation is a critical parameter in process control because it can directly affect the efficacy of ADC. Therefore, the level of unconjugated antibody is an important quality attribute that must monitored throughout the shelf life of the product. Creative Proteomics offers a wide range of assays based on different principles for the quantification of uncoupled antibodies in ADCs and provides complete data analysis. Our expertise and cutting-edge technologies enable us to provide accurate and reliable data to facilitate the characterization and development of antibody-coupled drugs.
Unconjugated Antibody Determination Services at Creative Proteomics
Creative Proteomics offers a range of innovative methods and strategies for the detection of uncoupled antibodies in ADC (Antibody Drug Conjugate) drugs. By working closely with our experienced team of scientists, we help our clients design and develop custom assays tailored to their specific demands.
Mass Spectrometry
Mass spectrometry is a powerful and versatile tool that has been successfully applied to the precise quantification of unconjugated antibodies. Our state-of-the-art platform equipped with a high-resolution mass spectrometer helps our clients to comprehensively analyze the percentage of unconjugated antibodies. Our skilled detection experts calculate the percentage of unconjugated antibodies based on the area under the curve of each peak and the total peak area, resulting in a complete characterization of the ADC.
Imaged Capillary Isoelectric Focusing (iCIEF)
As the conjugation of drug molecules onto the antibody can greatly alter the surface and overall electrostatic profile, we use the charge-based iCIEF technique to detect unconjugated antibody. ICIEF can accurately separate charge variants based on the isoelectric point (pI) of the charge. We separated antibodies from various ADC species based on the fact that unconjugated and drug-conjugated antibodies have different pI values and quantified them by iCIEF. In addition, our iCIEF technology can be used as a fast and convenient fingerprinting technique for ADCs to monitor any conjugation- or process-related changes.
Hydrophobic interaction chromatography (HIC)
The conjugation of a (hydrophobic) drug alters the hydrophobicity of the protein molecules due to the inherent lipophilic nature of the drug or linker. Creative Proteomics offers HIC-based technology to help customers determine the level of unconjugated antibodies in ADC samples. Unconjugated antibodies with minimal hydrophobicity elute first during the assay, followed by ADC molecules with increased surface hydrophobicity. Our experienced scientists adjust the retention and elution conditions according to the nature of the different samples to better separate the unconjugated antibodies from the coupled ones and calculate the level of unconjugated antibodies.
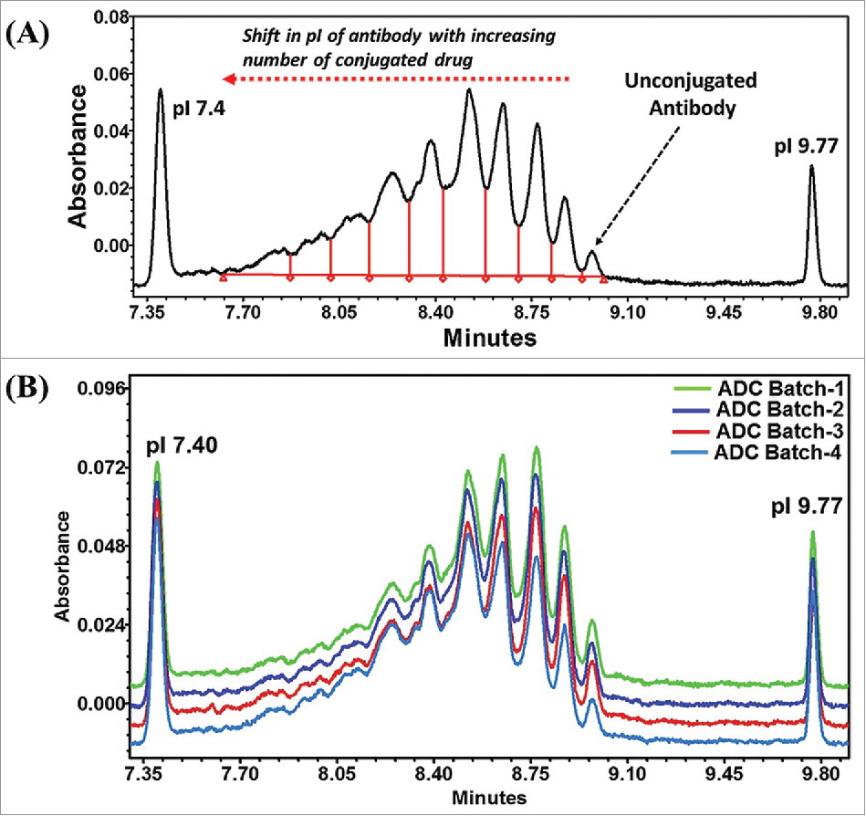 Fig. 1 (A) Electropherogram of a model Lysine based ADC. The amount of unconjugated antibody was determined by using calibration curve. (B) ICIEF as a finger printing technique to monitor the batch-to-batch process consistency. (Wagh, A., et al., 2018)
Fig. 1 (A) Electropherogram of a model Lysine based ADC. The amount of unconjugated antibody was determined by using calibration curve. (B) ICIEF as a finger printing technique to monitor the batch-to-batch process consistency. (Wagh, A., et al., 2018)
Advantages of Us
- Customizable solutions
- Expertise and experience
- Rapid turnaround time
Creative Proteomics' unconjugated antibody detection service utilizes advanced technology and cutting-edge techniques to evaluate unconjugated antibodies with high precision. Contact us to learn more about our service and we will be happy to serve you.
Reference
- Wagh, A.; et al. Challenges and new frontiers in analytical characterization of antibody-drug conjugates[C]//MAbs. Taylor & Francis. 2018, 10(2): 222-243.

 Fig. 1 (A) Electropherogram of a model Lysine based ADC. The amount of unconjugated antibody was determined by using calibration curve. (B) ICIEF as a finger printing technique to monitor the batch-to-batch process consistency. (Wagh, A., et al., 2018)
Fig. 1 (A) Electropherogram of a model Lysine based ADC. The amount of unconjugated antibody was determined by using calibration curve. (B) ICIEF as a finger printing technique to monitor the batch-to-batch process consistency. (Wagh, A., et al., 2018)