sdAb-Like Antibody Fragment
Online Inquiry
Since the first single-domain antibody therapeutics entered the clinical stage, the development of single-domain antibodies has been more than 10 years, both in depth and breadth. Single-domain antibodies have multiple advantages over traditional antibodies, making them useful as popular antibody drugs. Creative Proteomics provides customized characterization services for different single domain antibody drugs. With our extensive expertise and advanced characterization technologies, we can provide comprehensive and integrated characterization results to support the development and optimization of sdAb drugs.
Introduction of Single Domain Antibodies
Single domain antibody (sdAb) is a class of antibody that lacks the light chain of the antibody and only has the variable region of the heavy chain, and it is also called Nanobody because of its small molecular weight. Compared with traditional antibodies, single domain antibodies have the advantages of small molecular weight, high stability, and easy recombinant expression. Currently, therapeutic single-domain antibodies are often designed with multi-specific molecular linkages, structural modifications, or fusion proteins to extend the half-life and specificity in vivo. sdAb are emerging as a new class of antibody drugs that will bring more and better solutions to many diseases.
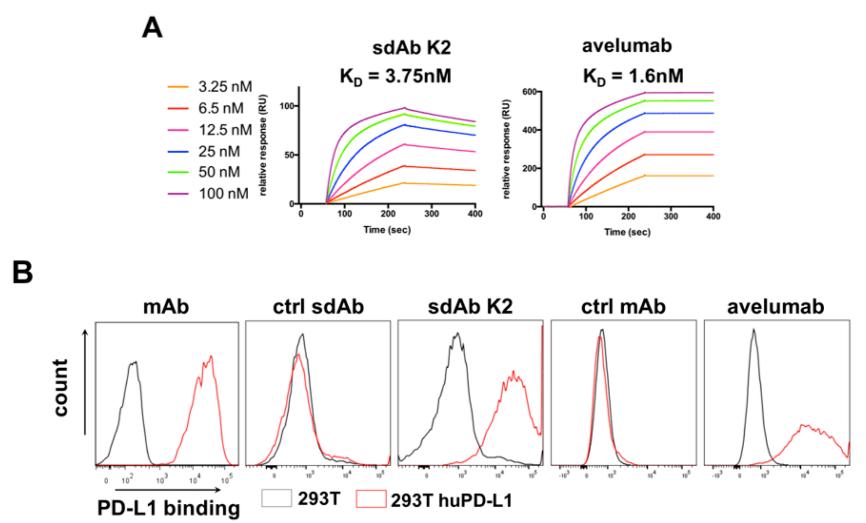 Fig. 1 Characterization of sdAb K2, a sdAb that specifically binds to human PD-L1. (Broos, K. et al., 2022)
Fig. 1 Characterization of sdAb K2, a sdAb that specifically binds to human PD-L1. (Broos, K. et al., 2022)
sdAb-Like Antibody Fragment Characterization Services at Creative Proteomics
Our sdAb drug characterization services are designed to provide comprehensive analysis of sdAb-based therapies to ensure their quality and efficacy. Our extensive characterization services include, but are not limited to, the following:
- Structural and physicochemical characterization: single domain antibody drug forms are highly expandable and can be combined with each other to form multivalent molecules, multi-specific molecules or with other compounds to form drug carriers. We provide a series of advanced analytical techniques to comprehensively analyze the molecular weight, primary structure, higher order structure, molecular polymerization, coupling or structural modification of single domain antibody drugs for different drug properties to ensure their quality and safety.
- Binding activity assessment: we employ a range of advanced analytical techniques to identify the binding sites of sdAb drugs on target molecules and analyze their binding affinity for the target. This information helps to assess drug specificity, biological activity, and potential cross-reactivity, providing valuable insights for the optimization and development of sdAb-based antibody drugs.
- Immunogenicity assessment: the potential immunogenicity of single domain antibody drugs is assessed by in vivo and in vitro experiments to ensure their safety.
- Stability evaluation: we conduct forced degradation experiments and long-term stability experiments under different temperatures, pH and other stress conditions to fully characterize the stability of single domain antibody drugs. Our accurate characterization results can provide customers with information on storage conditions and formulation optimization.
Characterization Service Process
At Creative Proteomics, you can get comprehensive small molecule antibody drug characterization services by following these simple steps.
To support the development of single domain antibody drugs, Creative Proteomics has established a comprehensive antibody drug characterization platform. Our customized characterization services provide comprehensive information on drug quality, safety and stability. Contact us for more information and we will be happy to assist you.
Reference
- Broos, K.; et al. Evaluating a single domain antibody targeting human PD-L1 as a nuclear imaging and therapeutic agent. Cancers. 2019, 11(6): 872.

 Fig. 1 Characterization of sdAb K2, a sdAb that specifically binds to human PD-L1. (Broos, K. et al., 2022)
Fig. 1 Characterization of sdAb K2, a sdAb that specifically binds to human PD-L1. (Broos, K. et al., 2022)