Glycosylation is an important post-translational modification of proteins. Glycosylation of therapeutic antibodies has far-reaching implications for their safety and efficacy. Creative Proteomics has developed a variety of analytical techniques to characterize issues related to different aspects of glycosylation. Our high-resolution analytical instrumentation combined with extensive expertise allows for comprehensive glycosylation analysis at different levels.
What is Glycosylation?
Glycosylation is a post-translational modification process in which a sugar molecule, or glycan, is attached to a protein or lipid molecule. This process typically occurs in the endoplasmic reticulum and Golgi apparatus of eukaryotic cells. There are different types of glycosylation, including N-glycosylation and O-glycosylation. In N-glycosylation, a glycan is attached to the side chain of an asparagine residue in a protein. In O-glycosylation, a glycan is attached to the hydroxyl group of a serine, threonine, or hydroxylysine residue.
Impact of Glycosylation on Antibody Characteristics
Different glycan chains affect the function of the Fc-effector and its half-life in serum. In particular, the lack of core fucoidan glycosylation enhances its affinity for the FcγRIIIa receptor, thereby increasing ADCC activity; the presence of α-2,6-sialic acid (i.e., N-acetylneuraminic acid, Neu5Ac) facilitates the enhancement of its anti-inflammatory activity. Wild-type mAb generally contains three to five glycoforms, which are mainly influenced by the expressing cell line, and if non-human cell lines are used they may produce non-human-derived glycosylation, which may generate unwanted immune responses. Therefore, glycosylation modifications are often controlled as critical quality attributes (CQA) of therapeutic antibody.
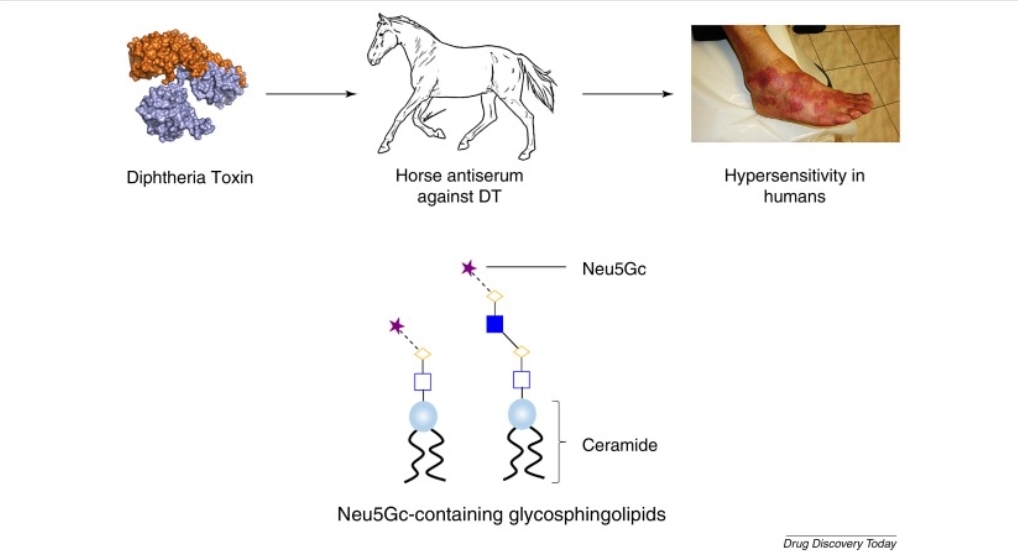 Fig. 1 The role of non-human sialic acid, Neu5Gc, in serum sickness. (Zhang, et al., 2016)
Fig. 1 The role of non-human sialic acid, Neu5Gc, in serum sickness. (Zhang, et al., 2016)
Our Glycan Analysis Services
Creative Proteomics has a variety of analytical methods available for characterization at the intact glycan level, including chromatography, electrophoresis, MS and other spectroscopic techniques. Our dedicated Characterization Services team provides comprehensive characterization services to provide data on different aspects of the glycan structure as required by the ICH guidelines, including:
- Identification of glycosylation sites through peptide mapping
- Determination of glycosylation linkage (GC-MS)
- Determination of Gu or G0 values (CE or normal phase LC)
- Determination of glycan structure (enzyme array, MALDI-TOF)
- Total sugar (monosaccharides and oligosaccharide composition neutral and amino sugars)
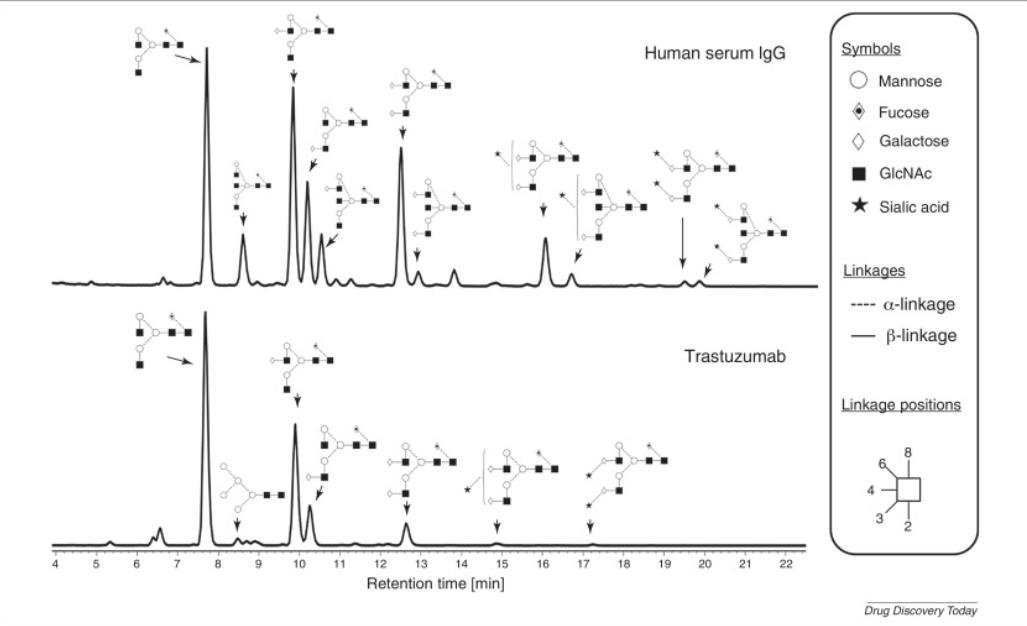 Fig. 2 Glycan profiles of human serum IgG and trastuzumab (Herceptin) analyzed by HILIC-UPLC. (Zhang, et al., 2016)
Fig. 2 Glycan profiles of human serum IgG and trastuzumab (Herceptin) analyzed by HILIC-UPLC. (Zhang, et al., 2016)
Our experts have extensive experience in this field. This enables us to provide you with customized characterization solutions based on your needs at different stages of development, helping you to optimize your manufacturing processes and enable your products to meet or exceed regulatory standards.
What We Deliver?
| Characterization scheme and process |
Liquid Chromatography and Mass Spectrometry Detection Parameters |
Mass Spectrometry Detection of Raw Data |
| Glycan analysis test results |
Bioinformatics Analysis Report |
|
Creative Proteomics requires comprehensive glycosylation characterization of glycoproteins according to ICH Q6B guidelines. For the complexity of structural characterization of glycosylation, we offer different methods and customized protocols in order to give you a full understanding of the diversity of glycoforms of these proteins, as well as an understanding of the structure of the glycans to avoid the risks associated with product safety and loss of activity. Contact us to learn more about our glycan analysis services of antibody drugs. We will be happy to assist you.
Reference
- Zhang, P.; et al. Challenges of glycosylation analysis and control: an integrated approach to producing optimal and consistent therapeutic drugs. Drug Discov Today. 2016 May;21(5):740-65.

 Fig. 1 The role of non-human sialic acid, Neu5Gc, in serum sickness. (Zhang, et al., 2016)
Fig. 1 The role of non-human sialic acid, Neu5Gc, in serum sickness. (Zhang, et al., 2016) Fig. 2 Glycan profiles of human serum IgG and trastuzumab (Herceptin) analyzed by HILIC-UPLC. (Zhang, et al., 2016)
Fig. 2 Glycan profiles of human serum IgG and trastuzumab (Herceptin) analyzed by HILIC-UPLC. (Zhang, et al., 2016)