Fourier Transform Infrared (FT-IR) Spectroscopy
Online Inquiry
Fourier Transform Infrared Spectroscopy (FT-IR) is a powerful analytical technique that is critical for gaining insight into the therapeutic behavior and stability of antibody drugs, as well as for optimizing the manufacturing process by serving as a quality control measure. Creative Proteomics offers advanced FT-IR spectroscopy technology that allows for accurate and precise analysis of the structural and functional properties of antibody drugs.
Introduction of Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR can provide detailed information about the structure and composition of antibody molecules by measuring the interaction between infrared radiation and molecular vibrations within the sample. FT-IR works on the principle that when a sample is exposed to an infrared light source, it absorbs specific wavelengths of light that are characteristic of its molecular vibrations. These absorption wavelengths correspond to the different functional groups present in the molecule (e.g., amide bonds, carbonyl and hydroxyl groups). By analyzing the specific wavelengths absorbed by an antibody drug, we can deduce valuable information about its structure and properties.
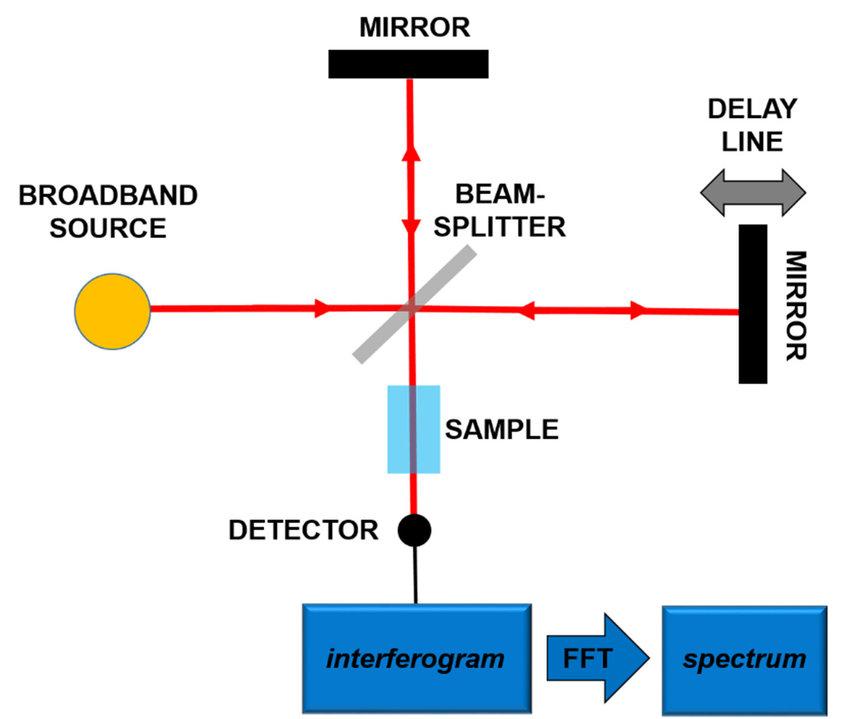 Fig. 1 Principles of the fourier-transform infrared spectroscopy (FT-IR). (Patrizi, B. et al., 2019)
Fig. 1 Principles of the fourier-transform infrared spectroscopy (FT-IR). (Patrizi, B. et al., 2019)
Application of FT-IR in Antibody Drug Characterization
Higher Order Structure Identification
Secondary/tertiary structure analysis is one of the main applications of FT-IR in the characterization of antibody drugs. FT-IR analysis produces a series of characteristic IR absorption bands. The amide I and II bands are the two most important vibrational bands of the protein backbone. Among them, the intensity and position changes of the amide I band are closely related to the secondary structure characteristics of proteins.
Aggregate or Metabolite Analysis
Protein aggregation is an important issue in antibody drug development, affecting drug efficacy and leading to immunogenic reactions. Fourier transform infrared (FT-IR) spectroscopy allows for the accurate identification and quantification of different forms of aggregates during drug storage, formulation and manufacturing processes.
Post-translational Modification Analysis
Fourier transform infrared (FT-IR) spectroscopy accurately evaluates the levels of phosphorylation and glycosylation in antibody drugs by detecting and quantifying the characteristic vibrational modes associated with PTM, thus effectively assessing the function and efficacy of antibody drugs.
Stability Analysis
By analyzing specific peaks in FTIR spectra, structural changes caused by factors such as pH, temperature, or chemical modifications can be monitored. This information is critical to understanding the stability and functionality of antibody drugs.
Advantages of Our Fourier Transform Infrared Spectroscopy (FT-IR)
- High sensitivity
- A label-free, non-destructive technique
- Fast data acquisition with minimal sample preparation
- Quantitative analysis
Our Capabilities
- Sensitivity, accuracy, and resolution of fourier transform infrared (FT-IR) spectroscopy analytical techniques.
- FTIR spectroscopy-based antibody drug characterization services, including secondary/tertiary structure characterization, denaturation and aggregation, prediction of metabolites, concentration, and to quantify levels of phosphorylation and glycosylation.
- Customized characterization protocols and expert data interpretation.
Creative Proteomics draws on our expertise in FT-IR technology to help accelerate the development and optimization of antibody drugs by providing customers with information about their molecular structure and behavior. Whether you need support with formulation development, stability studies, or batch-to-batch consistency analysis, we can help you in a timely manner. Contact us to learn more about our service and we will be happy to serve you.
Reference
- Patrizi, B.; et al. Dioxin and related compound detection: Perspectives for optical monitoring. International Journal of Molecular Sciences. 2019, 20(11): 2671.

 Fig. 1 Principles of the fourier-transform infrared spectroscopy (FT-IR). (Patrizi, B. et al., 2019)
Fig. 1 Principles of the fourier-transform infrared spectroscopy (FT-IR). (Patrizi, B. et al., 2019)